Controlled Lactonization of o-Coumaric Esters Mediated by Supramolecular Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Profragrances Preparation
2.2. Gel Characterization
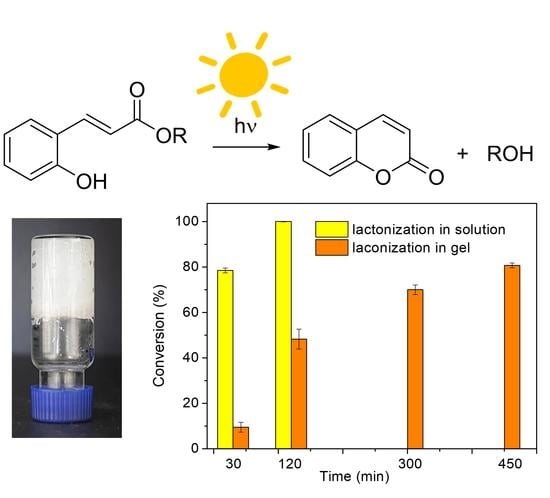
2.3. Controlled Cleavage of Profragrances A–D
3. Conclusions
4. Materials and Methods
4.1. General Remarks for the Synthetic Procedure
4.2. General Method for the Preparation of Profragrances A–D
4.3. Gel Preparation
4.4. IR Analysis of the Gels Samples
4.5. Optical Microscope Analysis
4.6. Rheological Analysis
4.7. SEM Analysis
4.8. X-ray Powder Diffraction Analysis
4.9. Solar Lamp Details
4.10. HPLC Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783540493389. [Google Scholar]
- The Chemistry of Fragrances: From Perfumer to Consumer, 2nd ed.; Sell, C.S. (Ed.) RSC Publishing: London, UK, 2006. [Google Scholar]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley-VCH: Weinheim, Germany, 2016; ISBN 9783527693153. [Google Scholar]
- Armanino, N.; Charpentier, J.; Flachsmann, F.; Goeke, A.; Liniger, M.; Kraft, P. What’s Hot, What’s Not: The Trends of the Past 20 Years in the Chemistry of Odorants. Angew. Chem. Int. Ed. 2020, 59, 16310–16344. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Bajgrowicz, J.A.; Denis, C.; Fráter, G. Odds and Trends: Recent Developments in the Chemistry of Odorants. Angew. Chem. Int. Ed. 2000, 39, 2980–3010. [Google Scholar] [CrossRef]
- Valls, A.; Castillo, A.; Porcar, R.; Hietala, S.; Altava, B.; García-Verdugo, E.; Luis, S.V. Urea-Based Low-Molecular-Weight Pseudopeptidic Organogelators for the Encapsulation and Slow Release of (R)-Limonene. J. Agric. Food Chem. 2020, 68, 7051–7061. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K. Handbook of Encapsulation and Controlled Release; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482232325. [Google Scholar]
- Ciriminna, R.; Pagliaro, M. Sol–gel microencapsulation of odorants and flavors: Opening the route to sustainable fragrances and aromas. Chem. Soc. Rev. 2013, 42, 9243–9250. [Google Scholar] [CrossRef]
- Ghosh, S.; Praveen, V.K.; Ajayaghosh, A. The Chemistry and Applications of π-Gels. Annu. Rev. Mater. Res. 2016, 46, 235–262. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Giuri, D.; Barbalinardo, M.; Zanna, N.; Paci, P.; Montalti, M.; Cavallini, M.; Valle, F.; Calvaresi, M.; Tomasini, C. Tuning Mechanical Properties of Pseudopeptide Supramolecular Hydrogels by Graphene Doping. Molecules 2019, 24, 4345. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Ravarino, P.; Di Domenico, N.; Barbalinardo, M.; Faccio, D.; Falini, G.; Giuri, D.; Tomasini, C. Fluorine Effect in the Gelation Ability of Low Molecular Weight Gelators. Gels 2022, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.M.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Ghosh, G.; Barman, R.; Sarkar, J.; Ghosh, S. pH-Responsive Biocompatible Supramolecular Peptide Hydrogel. J. Phys. Chem. B 2019, 123, 5909–5915. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Nicastro, G.; Black, L.M.; Ravarino, P.; D’agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef]
- Wei, M.; Pan, X.; Rong, L.; Dong, A.; He, Y.; Song, X.; Li, J. Polymer carriers for controlled fragrance release. Mater. Res. Express 2020, 7, 082001. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, Y.; Liu, Y.; Zhou, Z.; Shen, Y.; Jiang, L. Self-Assembling Oligo(2-oxazoline) Organogelators for the Encapsulation and Slow Release of Bioactive Volatiles. ACS Omega 2022, 7, 27523–27531. [Google Scholar] [CrossRef]
- Bratovčić, A. Synthesis of Gel Air Freshener and Its Stability. Technol. Acta 2019, 12, 15–21. [Google Scholar] [CrossRef]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, C.-H.; Abbaspourrad, A.; Wesner, C.; Caggioni, M.; Zhu, T.; Weitz, D.A. Encapsulation and Enhanced Retention of Fragrance in Polymer Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, A.G.; Hinze, O.; Görner, H.; Huchel, U.; Kropf, C.; Sundermeier, U.; Gerke, T. Aromatic aldols and 1,5-diketones as optimized fragrance photocages. Photochem. Photobiol. Sci. 2012, 11, 587–592. [Google Scholar] [CrossRef]
- Zhao, D.; Jiao, X.; Zhang, M.; Ye, K.; Shi, X.; Lu, X.; Qiu, G.; Shea, K.J. Preparation of high encapsulation efficiency fragrance microcapsules and their application in textiles. RSC Adv. 2016, 6, 80924–80933. [Google Scholar] [CrossRef]
- Hofmeister, I.; Landfester, K.; Taden, A. pH-Sensitive Nanocapsules with Barrier Properties: Fragrance Encapsulation and Controlled Release. Macromolecules 2014, 47, 5768–5773. [Google Scholar] [CrossRef]
- Günay, K.A.; Berthier, D.L.; Jerri, H.A.; Benczédi, D.; Klok, H.-A.; Herrmann, A. Selective Peptide-Mediated Enhanced Deposition of Polymer Fragrance Delivery Systems on Human Hair. ACS Appl. Mater. Interfaces 2017, 9, 24238–24249. [Google Scholar] [CrossRef]
- Tekin, R.; Bac, N.; Erdogmus, H. Microencapsulation of Fragrance and Natural Volatile Oils for Application in Cosmetics, and Household Cleaning Products. Macromol. Symp. 2013, 333, 35–40. [Google Scholar] [CrossRef]
- Herrmann, A. Controlled Release of Volatiles under Mild Reaction Conditions: From Nature to Everyday Products. Angew. Chem. Int. Ed. 2007, 46, 5836–5863. [Google Scholar] [CrossRef]
- Herrmann, A. Profragrance Chemistry as an Interdisciplinary Research Area and Key Technology for Fragrance Delivery. Chimia 2017, 71, 414–419. [Google Scholar] [CrossRef]
- Herrmann, A. Controlled release of volatile compounds using the Norrish type II reaction. Photochemistry 2019, 46, 242–264. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.; Alajarin, M.; Pastor, A.; Berna, J. Mechanically Interlocked Profragrances for the Controlled Release of Scents. J. Org. Chem. 2021, 86, 15045–15054. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yan, C.; Han, J.; Guo, Z.; Zhu, W.; Xiao, Z.; Wu, Y.; Huang, J. pH-activated polymeric profragrances for dual-controllable perfume release. AIChE J. 2021, 67, 8–16. [Google Scholar] [CrossRef]
- Irawan, C.; Nur, L.; Mellisani, B.; Arinzani. Hanafi Synthesis and characterization of citral-methylanthranilate schiff base, relationship between synthesis time and some physical properties. Rasayan J. Chem. 2019, 12, 951–958. [Google Scholar] [CrossRef]
- Irawan, C.; Islamiyati, D.; Utami, A.; Putri, I.D.; Putri, R.P.; Wibowo, S. Aurantiol Schiff base as A Raw Material in Fragrance Industry Synthesized by Simple Condensation Method and Its Characterization Using GC-MS. Orient. J. Chem. 2020, 36, 577–580. [Google Scholar] [CrossRef]
- Barmatov, E.; Hughes, T. Degradation of a Schiff-Base corrosion inhibitor by hydrolysis, and its effects on the inhibition efficiency for steel in hydrochloric acid. Mater. Chem. Phys. 2021, 257, 123758. [Google Scholar] [CrossRef]
- Indradas, B.; Hansen, C.; Palmer, M.; Womack, G.B. Autoxidation as a trigger for the slow release of volatile perfumery chemicals. Flavour Fragr. J. 2014, 29, 313–323. [Google Scholar] [CrossRef]
- Yang, Y.; Wahler, D.; Reymond, J.-L. β-Amino Alcohol Properfumes. Helvetica Chim. Acta 2003, 86, 2928–2936. [Google Scholar] [CrossRef]
- Derrera, S.; Flachsmann, F.; Plessis, C. Melanie Stang Applied Photochemistry—Light Controlled Perfume Release. Chimia 2007, 61, 665–669. [Google Scholar] [CrossRef]
- Nishimura, K.; Takenaka, Y.; Kishi, M.; Tanahashi, T.; Yoshida, H.; Okuda, C.; Mizushina, Y. Synthesis and DNA Polymerase a a and b b Inhibitory Activity of Alkyl P-Coumarates and Related Compounds Chart 1. Chem. Pharm. Bull. 2009, 57, 476–480. [Google Scholar] [CrossRef]
- Zanna, N.; Iaculli, D.; Tomasini, C. The effect ofl-DOPA hydroxyl groups on the formation of supramolecular hydrogels. Org. Biomol. Chem. 2017, 15, 5797–5804. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, A.; Dutot, L.; Barbeau, O.; Hamchaoui, W.; Wakselman, M.; Mazaleyrat, J.-P. Synthesis of terminally protected (S)-β3-H-DOPA by Arndt–Eistert homologation: An approach to crowned β-peptides. Tetrahedron Asymmetry 2005, 16, 857–864. [Google Scholar] [CrossRef]
- Giuri, D.; Ravarino, P.; Tomasini, C. l-Dopa in small peptides: An amazing functionality to form supramolecular materials. Org. Biomol. Chem. 2021, 19, 4622–4636. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-assembling of fibers inside an injectable calcium phosphate bone cement: A feasibility study. Mater. Today Chem. 2022, 24, 100991. [Google Scholar] [CrossRef]
- Toronyi, A.; Giuri, D.; Martiniakova, S.; Tomasini, C. Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics 2023, 10, 38. [Google Scholar] [CrossRef]
- Pour, S.R.S.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. [Google Scholar] [CrossRef]
- Suzuki, M.; Yumoto, M.; Shirai, H.; Hanabusa, K. Supramolecular Gels Formed by Amphiphilic Low-Molecular-Weight Gelators ofNα,Nɛ-Diacyl-L-Lysine Derivatives. Chem. A Eur. J. 2008, 14, 2133–2144. [Google Scholar] [CrossRef]
- Denzer, B.R.; Kulchar, R.J.; Huang, R.B.; Patterson, J. Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels 2021, 7, 158. [Google Scholar] [CrossRef]
- Adhikari, B.; Palui, G.; Banerjee, A. Self-assembling tripeptide based hydrogels and their use in removal of dyes from waste-water. Soft Matter 2009, 5, 3452–3460. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cenciarelli, F.; Falini, G.; Giuri, D.; Tomasini, C. Controlled Lactonization of o-Coumaric Esters Mediated by Supramolecular Gels. Gels 2023, 9, 350. https://doi.org/10.3390/gels9040350
Cenciarelli F, Falini G, Giuri D, Tomasini C. Controlled Lactonization of o-Coumaric Esters Mediated by Supramolecular Gels. Gels. 2023; 9(4):350. https://doi.org/10.3390/gels9040350
Chicago/Turabian StyleCenciarelli, Fabia, Giuseppe Falini, Demetra Giuri, and Claudia Tomasini. 2023. "Controlled Lactonization of o-Coumaric Esters Mediated by Supramolecular Gels" Gels 9, no. 4: 350. https://doi.org/10.3390/gels9040350
APA StyleCenciarelli, F., Falini, G., Giuri, D., & Tomasini, C. (2023). Controlled Lactonization of o-Coumaric Esters Mediated by Supramolecular Gels. Gels, 9(4), 350. https://doi.org/10.3390/gels9040350