Gel Formulations for Topical Treatment of Skin Cancer: A Review
Abstract
:1. Introduction
2. Systematic Search
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Drug Delivery Hurdles in Skin Cancer Treatment
3.1. Skin Structure
3.2. Skin Penetration Routes and Factors Influencing Skin Penetration
3.3. Opportunities for Increasing Skin Penetration
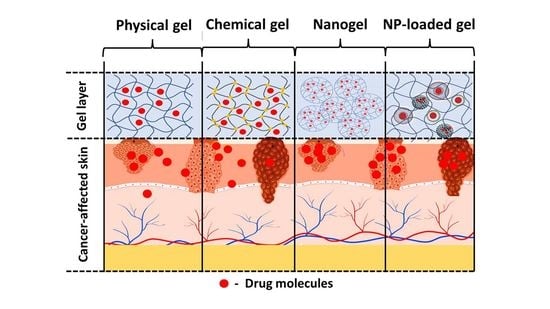
4. Gel-Based Formulations
4.1. Physical Hydrogels
4.1.1. Carbomer as a Gelling Agent
4.1.2. Cellulose Derivatives as a Gelling Agent
4.1.3. Poloxamer as Gelling Agent
4.1.4. Other Physical Hydrogels
4.2. Chemical Hydrogels
| Polymer | Cross-Linker | API | Method of Preparation | Ref. |
|---|---|---|---|---|
| Gelatin | Metacrylic anhydride | 5-fluorouracil | UV cross-linking | [115] |
| CMC | Citric acid | Doxorubicin | “green” method | [9,114] |
| Citric acid | Doxorubicin | “green” method | [114] | |
| METAC 1, DEGDMA 2 | curcumin | Free radical polymerization | [116] | |
| Carboxymethyl chitosan | Glutaraldehyde | 5-fluorouracil | Gelled deoxycholic acid micelles | [117] |
| Poly(acrylamide-co-diallyldimethylammonium chloride) | BisAA 3, APS 4/TEMED 5 | Indocyanine green | Free radical polymerization | [118] |
| Chitosan | PEGDA 6 | Aloe vera juice | UV cross-linking | [119] |
4.3. Nanogels
4.4. Nanocarrier-Loaded Gels
4.4.1. Nanovesicles
4.4.2. Lipid Nanoparticles
4.4.3. Inorganic Nanoparticles
4.4.4. Other Nanocarriers
4.5. Other Gels
5. Methods for Characterization of Gels
| Type of Characterization | Method | Application |
|---|---|---|
| Physico-chemical characterization | X-ray | The method is used for determination of the crystalline structure of polymers including crystal size, crystalline phases and their amount, crystallinity level and texture [177,178,179]. |
| pH | Gel sample is diluted in purified water to obtain a concentration of 10% w/v. A potentiometric measurement of the pH could be used to elucidate skin tolerability and possible stability issues [106]. | |
| UV-vis spectroscopy | It can be a useful tool for studying functional groups present in chemical gels, or molecular arrangement, such as π stacking, between aromatic rings in physical gels [179,180,181]. It can be also applied for the determination of lower critical solution temperature (LCST) related to the sol–gel transition properties [182] | |
| Infrared (IR) spectroscopy | IR spectroscopy is one of the most used confirmation methods to prove the structure of newly synthesized or already known polymers as a basis for gel preparation [13,17,18]. New functional groups or their absence are accounted for based on the bond energies that can be determined by the method [180] | |
| Nuclear magnetic resonance (NMR) | The specific transitions detected in the peaks can imply what kind and how many atoms are there in the structure. The chemical shifts are dependent on temperature and concentration, and thus, the NMR technique can be applied in gel characterization. Molecules come closer together in more concentrated solutions and this fact can be used to investigate the sol–gel transition in gel formulations [183] | |
| Thermal characterization | Thermogravimetric analysis (TGA) | In case of gels this method presents information regarding the thermal stability and the phase transitions of the gels [184,185]. |
| Differential scanning calorimetry (DSC) | It can be used to determine compatibility between various polymer blends, interaction of the gelling agent with the API, thermal stability, etc. [99]. It can be also applied to determine the interaction of the skin with the formulation as the thermogram of untreated skin shows one endothermic peak at 78 °C due to melting of stratum corneum lipid. If interaction is observed there would be a change in this peak [185]. | |
| Morphological characterization | Scanning electron microscopy (SEM) | Dried gel samples can be observed for their surface morphology at significant magnifications (300,000×). In addition, the high resolution allows detection of nanostructures with the gel base [186]. The data about surface roughness can be related to cell adherence and sustained drug release [186]. A confirmation of size could also be derived from this method [126] |
| Transmission electron microscopy (TEM) | The technique could be used in the chemical hydrogels and nanogels characterization, providing a very thin layer can be produced; otherwise, they are not visible on TEM [187]. | |
| Atomic force microscopy (AFM) | Direct observation of gel surface in water is possible providing specific conditions of the measurement are selected [188]. Usually the method is performed in non-contact mode for the evaluation of surface roughness. Furthermore, using force–distance curves, the elastic modulus of the sample can be determined. Adhesion to substrates (such as cells) can be another parameter determined by the method [189]. | |
| Mechanical characterization | Viscosimetry | Gel formation can be measured by monitoring their elastic (G′) and viscous (G″) moduli. When the value of G′ exceeds the one of G″, a gel is formed [190]. Furthermore, the gel kinetics can be observed [22]. |
| Texture analysis | The method allows evaluation of various parameters such as cohesiveness, adhesiveness, hardness, and extrudability [106,175]. They determine the ability to spread on the skin, the bioadhesion, and the ability to be evacuated from the packaging [106]. | |
| Spreadability | It is evaluated by the parallel plate method. A definite amount of the gel is placed on a glass plate. Another plate with known weight is positioned on top. After predetermined intervals additional weights are applied. The radius of spreading is measured and further used to calculate the spreadability factor [106,191,192]. | |
| Performance characterization | Swelling | Test for the ability of chemical hydrogels and nanogels [14] to imbibe water upon immersion in different liquid media (deionized water, phosphate buffer with various pH). The increase in weight of the gel sample over time is a measurement of its swelling ability. The results are usually directly related to the release of the loaded API [14,126] and the mechanical stability of the gel [114,193]. |
| Occlusion in vitro | A beaker is filled with water, covered by cellulose acetate filter, and sealed with Teflon tape. A predetermined amount of the gel formulation is evenly placed on top. These samples are kept in skin-mimicking conditions (temperature 32 °C) and constant humidity for 48 h. As a reference, a sample covered with filter paper only is used. The occlusion factor is calculated based on the change in weight of the samples [83,194,195,196]. | |
| Occlusion in vivo | The test is performed on healthy volunteers with an established protocol of application. At the beginning and after one week of application the skin hydration is determined based on capacity measurements with specific probes of Soft Plus apparatus (Callegari Srl, Parma, Italy) [196,197] | |
| In vitro release | This is an acellular assay in phosphate buffer with different pH [114] through a dialysis membrane [157] or with a membrane attached to a Franz-diffusion cell [137,143] which measures the amount of drug released over time. | |
| Permeation studies | Ex vivo hairless animal skin is used together with a Franz-diffusion cell to measure the amount of drug that passes through the membrane [38,123,124]. Artificial membranes can be applied such as Strat-M® membrane or keratinocytes culture (EpiDermTM) in a Franz-diffusion set-up [80]. They are standard and thus provide better reproducibility of the results [198,199]. | |
| Skin deposition studies | Confocal laser scanning microscopy (CLSM) is used in combination with permeation studies in order to evaluate the depth of penetration, cell internalization, and formulation factors affecting them. Usually, rhodamine B or other fluorescent dye is loaded in the tested sample. A substrate is treated with the gel formulation and optically scanned with fluorescent microscope. The substrate could present in vitro grown cells [156], excised skin sample used in a Franz-diffusion set-up [165,200], or in vivo observed skin [201] Tape stripping technique can non-invasively measure the amount of drug penetrated in the stratum corneum [201,202,203]. |
5.1. Physico-Chemical and Thermal Characterization Methods
5.2. Rheology Studies
5.3. Morphology Charcaterization
5.4. Performance Characterization
5.4.1. Occlusion
5.4.2. In Vitro Release
5.4.3. Drug Penetration
In Vivo Methods
Pharmacokinetic Studies
Skin Biopsy and Suction Blister Methods
Microdialysis
Tape Stripping
Confocal Laser Scanning Microscopy (CLSM)
In Vitro Methods
6. Conclusions and Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659. [Google Scholar] [CrossRef]
- Craythorne, E.; Al-Niami, F. Skin Cancer. Medicine 2017, 45, 431–434. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and Treatment of Basal Cell Carcinoma: European Consensus–Based Interdisciplinary Guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537. [Google Scholar] [CrossRef]
- Barrera, M.V.; Herrera, E. Topical Chemotherapy for Actinic Keratosis and Nonmelanoma Skin Cancer: Current Options and Future Perspectives. Actas Dermo-Sifiliográficas Engl. Ed. 2007, 98, 556–562. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol. Pharm. 2018, 15, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Delledonne, A.; Ferraboschi, I.; Sissa, C.; Terenziani, F.; De Freitas Rosa Remiro, P.; Santi, P.; Nicoli, S. Improvement of Imiquimod Solubilization and Skin Retention via Tpgs Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid. Pharmaceutics 2021, 13, 1476. [Google Scholar] [CrossRef]
- Lapteva, M.; Mignot, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-Assembled MPEG-HexPLA Polymeric Nanocarriers for the Targeted Cutaneous Delivery of Imiquimod. Eur. J. Pharm. Biopharm. 2019, 142, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Bioengineered Carboxymethyl Cellulose-Doxorubicin Prodrug Hydrogels for Topical Chemotherapy of Melanoma Skin Cancer. Carbohydr. Polym. 2018, 195, 401–412. [Google Scholar] [CrossRef]
- Gamal, F.A.; Sayed, O.M.; El-Ela, F.I.A.; Kharshoum, R.M.; Salem, H.F. Treatment of Basal Cell Carcinoma Via Binary Ethosomes of Vismodegib: In Vitro and In Vivo Studies. AAPS PharmSciTech 2020, 21, 51. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and Vismodegib in the Treatment of Patients with Locally Advanced Basal Cell Carcinoma: A Joint Expert Opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef]
- Mousa, I.A.; Hammady, T.M.; Gad, S.; Zaitone, S.A.; El-Sherbiny, M.; Sayed, O.M. Formulation and Characterization of Metformin-Loaded Ethosomes for Topical Application to Experimentally Induced Skin Cancer in Mice. Pharmaceuticals 2022, 15, 657. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.K.; Tallapaneni, V.; Sanapalli, B.K.R.; Kumar, G.V.; Karri, V.V.S.R. Curcumin Loaded Ethosomal Vesicular Drug Delivery System for the Treatment of Melanoma Skin Cancer. Res. J. Pharm. Technol. 2019, 12, 1783–1792. [Google Scholar] [CrossRef]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-Loaded Layer-by-Layer Folic Acid and Casein Coated Carboxymethyl Cellulose/Casein Nanogels for Treatment of Skin Cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Aldawsari, H.M.; Ali, J.; Gupta, D.K.; Warsi, M.H.; Bilgrami, A.L.; Asfour, H.Z.; Noor, A.O.; Md, S. Brucine-Loaded Transliposomes Nanogel for Topical Delivery in Skin Cancer: Statistical Optimization, in Vitro and Dermatokinetic Evaluation. 3 Biotech 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ali, J.; Ganguli, M.; Mishra, S.; Baboota, S. Silymarin-Loaded Nanostructured Lipid Carrier Gel for the Treatment of Skin Cancer. Nanomedicine 2019, 14, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical Nanoemulgel for the Treatment of Skin Cancer: Proof-of-Technology. Pharmaceutics 2021, 13, 902. [Google Scholar] [CrossRef]
- Kaplan, A.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Hekimoglu, S. Formulation and in Vitro Evaluation of Topical Nanoemulsion and Nanoemulsion-Based Gels Containing Daidzein. J. Drug Deliv. Sci. Technol. 2019, 52, 189–203. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-Delivery of Curcumin and STAT3 SiRNA Using Cationic Liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Gupta, P.; Garg, S. Semisolid Dosage Forms for Dermatological Application. Pharm. Technol. 2002, 3, 144–162. [Google Scholar]
- Un Nabi, S.A.A.; Sheraz, M.A.; Ahmed, S.; Mustaan, N.; Ahmad, I. Pharmaceutical Gels: A Review. RADS J. Pharm. Pharm. Sci. 2016, 4, 40–48. [Google Scholar]
- Goyal, N.; Thatai, P.; Sapra, B. Skin Cancer: Symptoms, Mechanistic Pathways and Treatment Rationale for Therapeutic Delivery. Ther. Deliv. 2017, 8, 265–287. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Pan, M.; Shi, K.; Hu, D.; Li, Y.; Chen, Y.; Qian, Z. Nanocarriers for Promoting Skin Delivery of Therapeutic Agents. Appl. Mater. Today 2022, 27, 101438. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahsan, M.J.; Rahman, M.; Anwar, S.; Rizwanullah, M. Advancement in Nanotheranostics for Effective Skin Cancer Therapy: State of the Art. Curr. Nanomed. 2020, 10, 90–104. [Google Scholar] [CrossRef]
- Farhana, A. Enhancing Skin Cancer Immunotheranostics and Precision Medicine through Functionalized Nanomodulators and Nanosensors: Recent Development and Prospects. Int. J. Mol. Sci. 2023, 24, 3493. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in Design of Nanostructured Lipid Carriers for Cancer Targeting and Theranostic Application. Biochim. Biophys. Acta BBA Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Shende, P.; Vaidya, J.; Gaud, R.S. Pharmacotherapeutic Approaches for Transportation of Anticancer Agents via Skin. Artif. Cells Nanomed. Biotechnol. 2018, 46, S423–S433. [Google Scholar] [CrossRef]
- Depieri, L.V.; Praça, F.S.G.; Campos, P.M.; Bentley, M.V.L.B. Advances in the Bioanalytical Study of Drug Delivery across the Skin. Ther. Deliv. 2015, 6, 571–594. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Khan, M.F.A.; Rehman, A.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin Cancer Biology and Barriers to Treatment: Recent Applications of Polymeric Micro/Nanostructures. J. Adv. Res. 2022, 36, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of Drugs through Skin, a Complex Rate-Controlling Membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current Status and Future Potential of Transdermal Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the Formation, Structure and Barrier Function of the Stratum Corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of Penetration Potential of PH Responsive Double Walled Biodegradable Nanogels Coated with Eucalyptus Oil for the Controlled Delivery of 5-Fluorouracil: In Vitro and Ex Vivo Studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Eady, R.A.J.; Pope, F.M. Anatomy and Organization of Human Skin. In Rook’s Textbook of Dermatology; Wiley: Hoboken, NJ, USA, 2004; Volume 1, Chapter 3; pp. 1–84. [Google Scholar]
- Taveira, S.F.; Lopez, R.F.V.; Taveira, S.F.; Lopez, R.F.V. Topical Administration of Anticancer Drugs for Skin Cancer Treatment; IntechOpen: London, UK, 2011; ISBN 978-953-307-722-2. [Google Scholar]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Iyer, A.K. Discovering PH Triggered Charge Rebound Surface Modulated Topical Nanotherapy against Aggressive Skin Papilloma. Mater. Sci. Eng. C 2020, 107, 110263. [Google Scholar] [CrossRef]
- Amasya, G.; Aksu, B.; Badilli, U.; Onay-Besikci, A.; Tarimci, N. QbD Guided Early Pharmaceutical Development Study: Production of Lipid Nanoparticles by High Pressure Homogenization for Skin Cancer Treatment. Int. J. Pharm. 2019, 563, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Williams, A. Transdermal and Topical Drug Delivery from Theory to Clinical Practice; Pharmaceutical Press: London, UK, 2003; ISBN 978-0-85369-489-2. [Google Scholar]
- National Research Council (US) Commission on Engineering and Technical Systems; Wartell, M.A.; Kleinman, M.T.; Huey, B.M. Strategies to Protect the Health of Deployed U.S. Forces: Force Protection and Decontamination. Washington (DC): National Academies Press (US); 1999. Appendix E, Percutaneous Absorption. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225150/ (accessed on 1 March 2023).
- Touitou, E.; Barry, B.W. (Eds.) Enhancement in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-12231-6. [Google Scholar]
- Taylor, K.M.G.; Wenande, E.; Olesen, U.H.; Nielsen, M.M.B.; Janfelt, C.; Hansen, S.H.; Anderson, R.R.; Haedersdal, M. Fractional Laser-Assisted Topical Delivery Leads to Enhanced, Accelerated and Deeper Cutaneous 5-Fluorouracil Uptake: Expert Opinion on Drug Delivery: Volume 14, No 3. Available online: https://www.tandfonline.com/doi/abs/10.1080/17425247.2017.1260119 (accessed on 3 March 2023).
- De Oliveira, B.E.; Amorim, O.H.J.; Lima, L.L.; Rezende, R.A.; Mestnik, N.C.; Bagatin, E.; Leonardi, G.R. 5-Fluorouracil, Innovative Drug Delivery Systems to Enhance Bioavailability for Topical Use. J. Drug Deliv. Sci. Technol. 2021, 61, 102155. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Love, W.E.; Bernhard, J.D.; Bordeaux, J.S. Topical Imiquimod or Fluorouracil Therapy for Basal and Squamous Cell Carcinoma: A Systematic Review. Arch. Dermatol. 2009, 145, 1431–1438. [Google Scholar] [CrossRef]
- Telò, I.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Mechanisms of Imiquimod Skin Penetration. Int. J. Pharm. 2016, 511, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayahy, M.H.; Sabri, A.H.; Rutland, C.S.; Holmes, A.; McKenna, J.; Marlow, M.; Scurr, D.J. Insight into Imiquimod Skin Permeation and Increased Delivery Using Microneedle Pre-Treatment. Eur. J. Pharm. Biopharm. 2019, 139, 33–43. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting Multidrug Resistance in Cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, N.; Kitajima, Y. Chemotherapy Induces or Increases Expression of Multidrug Resistance-Associated Protein in Malignant Melanoma Cells. Br. J. Dermatol. 2001, 144, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Grottke, C.; Mantwill, K.; Dietel, M.; Schadendorf, D.; Lage, H. Identification of Differentially Expressed Genes in Human Melanoma Cells with Acquired Resistance to Various Antineoplastic Drugs. Int. J. Cancer 2000, 88, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, M.; Bengala, C.; Conte, P.F.; Danova, M.; Pronzato, P.; Rosti, G.; Sagrada, P. Dose and Outcome: The Hurdle of Neutropenia (Review). Oncol. Rep. 2006, 16, 233–248. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a Bioactive Material to Regulate Stem Cell Fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Collaud, S.; Peng, Q.; Gurny, R.; Lange, N. Thermosetting Gel for the Delivery of 5-Aminolevulinic Acid Esters to the Cervix. J. Pharm. Sci. 2008, 97, 2680–2690. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-Sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered Hydrogels in Cancer Therapy and Diagnosis. Trends Biotechnol. 2017, 35, 1074–1087. [Google Scholar] [CrossRef]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and Characterization of Polyglycerol-Based Thermoresponsive Nanogels Designed as Novel Drug-Delivery Systems and Their Intracellular Localization in Keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.), 11th ed.; European Directorate for the Quality of Medicines & HealthCare—EDQM: Strasbourg, France, 2022; Available online: https://www.edqm.eu/en/ (accessed on 26 February 2023).
- Nayak, A.K.; Das, B. Introduction to Polymeric Gels. In Polymeric Gels: Characterization, Properties and Biomedical Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 3–27. ISBN 978-0-08-102179-8. [Google Scholar]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, L.; Yuan, J.; Chen, Y.; Leng, Y. Chapter 3—Physical Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–89. ISBN 978-0-12-816421-1. [Google Scholar]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An Overview of Hydrogels and Their Role in Transdermal Drug Delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Shaw, C. Chapter 5—Use of Polymers and Thickeners in Semisolid and Liquid Formulations. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Kulkarni, V.S., Shaw, C., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 43–69. ISBN 978-0-12-801024-2. [Google Scholar]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Chapter 7—Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 181–223. ISBN 978-0-12-802447-8. [Google Scholar]
- Lochhead, R.Y. The Use of Polymers in Cosmetic Products. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–221. ISBN 978-0-12-802005-0. [Google Scholar]
- Safitri, F.I.; Nawangsari, D.; Febrina, D. Overview: Application of Carbopol 940 in Gel; Atlantis Press: Paris, France, 2021; pp. 80–84. [Google Scholar]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, Mucoadhesive and Release Properties of Carbopol Gels in Hydrophilic Cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Varges, R.P.; Costa, M.C.; Fonseca, S.B.; Naccache, F.M.; De Souza Mendes, P.R. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Ruiz, V.H.; Encinas-Basurto, D.; Sun, B.; Eedara, B.B.; Dickinson, S.E.; Wondrak, G.T.; Chow, H.-H.S.; Curiel-Lewandrowski, C.; Mansour, H.M. Design, Physicochemical Characterization, and In Vitro Permeation of Innovative Resatorvid Topical Formulations for Targeted Skin Drug Delivery. Pharmaceutics 2022, 14, 700. [Google Scholar] [CrossRef]
- Osipitan, O.; Shi, Y.; Di Pasqua, A. Phenethyl Isothiocyanate-Containing Carbomer Gel for Use against Squamous Cell Carcinoma. Pharmaceutics 2021, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Abkin, S.V.; Pankratova, K.M.; Komarova, E.Y.; Guzhova, I.V.; Margulis, B.A. Hsp70 Chaperone-Based Gel Composition as a Novel Immunotherapeutic Anti-Tumor Tool. Cell Stress Chaperones 2013, 18, 391–396. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Haloi, J.; Medhi, S. Topical Delivery of Methanolic Root Extract of Annona Reticulata against Skin Cancer. S. Afr. J. Bot. 2019, 124, 484–493. [Google Scholar] [CrossRef]
- Merclin, N.; Bramer, T.; Edsman, K. Iontophoretic Delivery of 5-Aminolevulinic Acid and Its Methyl Ester Using a Carbopol Gel as Vehicle. J. Control. Release 2004, 98, 57–65. [Google Scholar] [CrossRef]
- Saez, V.; Khoury, H.J.; da Silva, M.I.B.; Mansur, C.R.E.; Santos-Oliveira, R. Rheological Effect of Gamma Radiation on Gel-like Formulation: Appraisal for the Construction of Radiopharmaceuticals for Cutaneous Application. Radiat. Phys. Chem. 2018, 145, 19–25. [Google Scholar] [CrossRef]
- Borghi-Pangoni, F.B.; Junqueira, M.V.; Ferreira, S.B.d.S.; Silva, L.L.; Rabello, B.R.; de Castro, L.V.; Baesso, M.L.; Diniz, A.; Caetano, W.; Bruschi, M.L. Preparation and Characterization of Bioadhesive System Containing Hypericin for Local Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 284–297. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.S.; Marchetti, J.M.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V.L.B. A Vehicle for Photodynamic Therapy of Skin Cancer: Influence of Dimethylsulphoxide on 5-Aminolevulinic Acid in Vitro Cutaneous Permeation and in Vivo Protoporphyrin IX Accumulation Determined by Confocal Microscopy. J. Control. Release 2000, 65, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose Hydrogels: Green and Sustainable Soft Biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Suflet, D.M. 11—Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–439. ISBN 978-0-444-63774-1. [Google Scholar]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Fahr, A.; Voigt, R. Voigt Pharmazeutische Technologie; Deutscher Apotheker Verlag: Stuttgart, Deutschland, 2021. [Google Scholar]
- Oprea, M.; Voicu, S.I. Recent Advances in Composites Based on Cellulose Derivatives for Biomedical Applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Ceschel, G.C.; Mora, P.C.; Borgia, S.L.; Maffei, P.; Ronchi, C. Skin Permeation Study of Dehydroepiandrosterone (DHEA) Compared with Its A-Cyclodextrin Complex Form. J. Pharm. Sci. 2002, 91, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Torky, A.S.; Freag, M.S.; Nasra, M.M.A.; Abdallah, O.Y. Novel Skin Penetrating Berberine Oleate Complex Capitalizing on Hydrophobic Ion Pairing Approach. Int. J. Pharm. 2018, 549, 76–86. [Google Scholar] [CrossRef]
- Doneda, E.; Bianchi, S.E.; Pittol, V.; Kreutz, T.; Scholl, J.N.; Ibañez, I.L.; Bracalente, C.; Durán, H.; Figueiró, F.; Klamt, F.; et al. 3-O-Methylquercetin from Achyrocline Satureioides—Cytotoxic Activity against A375-Derived Human Melanoma Cell Lines and Its Incorporation into Cyclodextrins-Hydrogels for Topical Administration. Drug Deliv. Transl. Res. 2021, 11, 2151–2168. [Google Scholar] [CrossRef]
- SreeHarsha, N.; Hiremath, J.; Rawre, B.; Puttaswamy, N.; Al-Dhubiab, B.; Venugopala, K.; Akrawi, S.; Meravanige, G.; Attimarad, M.; Nair, A. Formulation and Evaluation of Tamoxifen Citrate Loaded Transdermal Reservoir Gel Drug Delivery Systems. Indian J. Pharm. Educ. Res. 2019, 53, S596–S606. [Google Scholar] [CrossRef]
- Palem, R.R.; Rao, K.M.; Shimoga, G.; Saratale, R.G.; Shinde, S.K.; Ghodake, G.S.; Lee, S.-H. Physicochemical Characterization, Drug Release, and Biocompatibility Evaluation of Carboxymethyl Cellulose-Based Hydrogels Reinforced with Sepiolite Nanoclay. Int. J. Biol. Macromol. 2021, 178, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Tiossi, R.F.J.; Da Costa, J.C.; Miranda, M.A.; Praça, F.S.G.; McChesney, J.D.; Bentley, M.V.L.B.; Bastos, J.K. In Vitro and in Vivo Evaluation of the Delivery of Topical Formulations Containing Glycoalkaloids of Solanum Lycocarpum Fruits. Eur. J. Pharm. Biopharm. 2014, 88, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guan, J. Thermosensitive Hydrogels for Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 991–1007. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Wang, L.; Wang, S.; Georgiou, T.K. Thermoresponsive Block Copolymers of Increasing Architecture Complexity: A Review on Structure–Property Relationships. Polym. Chem. 2023, 14, 223–247. [Google Scholar] [CrossRef]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal Delivery of the in situ Hydrogels of Curcumin and Its Inclusion Complexes of Hydroxypropyl-β-Cyclodextrin for Melanoma Treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Redpath, M.; Marques, C.M.G.; Dibden, C.; Waddon, A.; Lalla, R.; MacNeil, S. Ibuprofen and Hydrogel-Released Ibuprofen in the Reduction of Inflammation-Induced Migration in Melanoma Cells. Br. J. Dermatol. 2009, 161, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Takeuchi, S.; Yokouchi, C.; Takada, M. Pluronic F-127 Gels as a Vehicle for Topical Administration of Anticancer Agents1,2). Chem. Pharm. Bull. 1984, 32, 4205–4208. [Google Scholar] [CrossRef]
- Batista, C.M.; de Queiroz, L.A.; Alves, Â.V.F.; Reis, E.C.A.; Santos, F.A.; Castro, T.N.; Lima, B.S.; Araújo, A.A.S.; Godoy, C.A.P.; Severino, P.; et al. Photoprotection and Skin Irritation Effect of Hydrogels Containing Hydroalcoholic Extract of Red Propolis: A Natural Pathway against Skin Cancer. Heliyon 2022, 8, e08893. [Google Scholar] [CrossRef]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive Polymeric Hydrogels as Drug Delivery Systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef]
- Campanholi, K.D.S.S.; Braga, G.; Da Silva, J.B.; Da Rocha, N.L.; De Francisco, L.M.B.; De Oliveira, É.L.; Bruschi, M.L.; De Castro-Hoshino, L.V.; Sato, F.; Hioka, N.; et al. Biomedical Platform Development of a Chlorophyll-Based Extract for Topic Photodynamic Therapy: Mechanical and Spectroscopic Properties. Langmuir 2018, 34, 8230–8244. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels Based on Cellulose and Chitin: Fabrication, Properties, and Applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Nair, A.; Nair, S.C.; Banerji, A.; Biswas, R.; Mony, U. Development and Evaluation of Plumbagin Loaded Chitin Hydrogel for the Treatment of Skin Cancer. J. Drug Deliv. Sci. Technol. 2021, 66, 102804. [Google Scholar] [CrossRef]
- Kochkina, N.; Nikitina, M.; Agafonov, M.; Delyagina, E.; Terekhova, I. Iota-Carrageenan Hydrogels for Methotrexate Delivery. J. Mol. Liq. 2022, 368, 120790. [Google Scholar] [CrossRef]
- Taktak, F.; Bütün, V.; Tuncer, C.; Demirel, H.H. Production of LMWH-Conjugated Core/Shell Hydrogels Encapsulating Paclitaxel for Transdermal Delivery: In Vitro and in Vivo Assessment. Int. J. Biol. Macromol. 2019, 128, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Carvalho, I.C.; Mansur, A.A.P.; Carvalho, S.M.; Lage, A.P.; Mansur, H.S. Hybrid Hydrogel Composed of Carboxymethylcellulose-Silver Nanoparticles-Doxorubicin for Anticancer and Antibacterial Therapies against Melanoma Skin Cancer Cells. ACS Appl. Nano Mater. 2019, 2, 7393–7408. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Mansur, A.A.P.; Capanema, N.S.V.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Synthesis and in Vitro Assessment of Anticancer Hydrogels Composed by Carboxymethylcellulose-Doxorubicin as Potential Transdermal Delivery Systems for Treatment of Skin Cancer. J. Mol. Liq. 2018, 266, 425–440. [Google Scholar] [CrossRef]
- Oktay, S.; Alemdar, N. Electrically Controlled Release of 5-Fluorouracil from Conductive Gelatin Methacryloyl-Based Hydrogels. J. Appl. Polym. Sci. 2019, 136, 46914. [Google Scholar] [CrossRef]
- Mandal, B.; Rameshbabu, A.P.; Soni, S.R.; Ghosh, A.; Dhara, S.; Pal, S. In Situ Silver Nanowire Deposited Cross-Linked Carboxymethyl Cellulose: A Potential Transdermal Anticancer Drug Carrier. ACS Appl. Mater. Interfaces 2017, 9, 36583–36595. [Google Scholar] [CrossRef]
- Pourmanouchehri, Z.; Ebrahimi, S.; Limoee, M.; Jalilian, F.; Janfaza, S.; Vosoughi, A.; Behbood, L. Controlled Release of 5-Fluorouracil to Melanoma Cells Using a Hydrogel/Micelle Composites Based on Deoxycholic Acid and Carboxymethyl Chitosan. Int. J. Biol. Macromol. 2022, 206, 159–166. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.-O. Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma. Biomolecules 2020, 10, 1124. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Głąb, M.; Drabczyk, A.; Kordyka, A.; Godzierz, M.; Wróbel, P.S.; Krzan, M.; Uthayakumar, M.; Kędzierska, M.; Tyliszczak, B. Physicochemical Characteristics of Chitosan-Based Hydrogels Containing Albumin Particles and Aloe Vera Juice as Transdermal Systems Functionalized in the Viewpoint of Potential Biomedical Applications. Materials 2021, 14, 5832. [Google Scholar] [CrossRef] [PubMed]
- Marzi, M.; Chijan, M.R.; Zarenezhad, E. Hydrogels as Promising Therapeutic Strategy for the Treatment of Skin Cancer. J. Mol. Struct. 2022, 1262, 133014. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tiwari, S. A Review on Biomacromolecular Hydrogel Classification and Its Applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. PH Triggered and Charge Attracted Nanogel for Simultaneous Evaluation of Penetration and Toxicity against Skin Cancer: In-Vitro and Ex-Vivo Study. Int. J. Biol. Macromol. 2019, 128, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, M.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and Evaluation of 5-Fluorouracil Loaded Chitin Nanogels for Treatment of Skin Cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Nair, A.; Sabitha, M.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, Characterization and in Vitro Cytocompatibility Studies of Chitin Nanogels for Biomedical Applications. Carbohydr. Polym. 2012, 87, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. PH Responsive Biodegradable Nanogels for Sustained Release of Bleomycin. Bioorg. Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef] [PubMed]
- Kamal, I.; Khedr, A.I.M.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Saad, A.S. Chemotherapeutic and Chemopreventive Potentials of ρ-Coumaric Acid—Squid Chitosan Nanogel Loaded with Syzygium Aromaticum Essential Oil. Int. J. Biol. Macromol. 2021, 188, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Pitorre, M.; Gondé, H.; Haury, C.; Messous, M.; Poilane, J.; Boudaud, D.; Kanber, E.; Ndombina, G.A.R.; Benoit, J.-P.; Bastiat, G. Recent Advances in Nanocarrier-Loaded Gels: Which Drug Delivery Technologies against which Diseases? J. Control. Release 2017, 266, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-J.; Larsson, M.; Liu, D.-M. A Novel Dual-Structure, Self-Healable, Polysaccharide Based Hybrid Nanogel for Biomedical Uses. Soft Matter 2011, 7, 5816–5825. [Google Scholar] [CrossRef]
- Manosroi, A.; Kongkaneramit, L.; Manosroi, J. Stability and Transdermal Absorption of Topical Amphotericin B Liposome Formulations. Int. J. Pharm. 2004, 270, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Ramzan, M.; Ahsan, M.N.; Ur Rehman, Z.; Ahmad, F.J. Elastic Liposome-Based Gel for Topical Delivery of 5-Fluorouracil: In Vitro and in Vivo Investigation. Drug Deliv. 2016, 23, 1115–1129. [Google Scholar] [CrossRef]
- Nekvasil, M.; Zadinova, M.; Tahotna, L.; Zackova, M.; Pouckova, P.; Jezek, P. Optimum Modality for Photodynamic Therapy of Tumors: Gels Containing Liposomes with Hydrophobic Photosensitizers. Drug Dev. Res. 2007, 68, 235–252. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Winter, S.; Krajisnik, D.; Stupar, M.; Milic, J.; Graefe, S.; Fahr, A. Stability Evaluation of Temoporfin-Loaded Liposomal Gels for Topical Application. J. Liposome Res. 2010, 20, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Marwah, M.; Badhan, R.K.S.; Lowry, D. Development of a Novel Polymer-Based Carrier for Deformable Liposomes for the Controlled Dermal Delivery of Naringenin. J. Liposome Res. 2021, 32, 181–194. [Google Scholar] [CrossRef]
- Famta, P.; Shah, S.; Fernandes, V.; Kumar, K.C.; Bagasariya, D.; Samim, K.S.; Khatri, D.K.; Singh, S.B.; Srivastava, S. Quality by Design (QbD) Assisted Fabrication & Evaluation of Simvastatin Loaded Nano-Enabled Thermogel for Melanoma Therapy. Int. J. Pharm. 2022, 628, 122270. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Paul, A.; Cevc, G.; Bachhawat, B.K. Transdermal Immunization with Large Proteins by Means of Ultradeformable Drug Carriers. Eur. J. Immunol. 1995, 25, 3521–3524. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sapre, R.; Umamaheswari, R.B.; Jain, N.K. Protransfersomes for Effective Transdermal Delivery of Norgestrel Preparation and in Vitro Characterization. Indian J. Pharm. Sci. 2003, 65, 152. [Google Scholar]
- Gupta, V.; Trivedi, P. Ex Vivo Localization and Permeation of Cisplatin from Novel Topical Formulations through Excised Pig, Goat, and Mice Skin and in Vitro Characterization for Effective Management of Skin-Cited Malignancies. Artif. Cells Nanomed. Biotechnol. 2015, 43, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel Carbopol-Based Transfersomal Gel of 5-Fluorouracil for Skin Cancer Treatment: In Vitro Characterization and in Vivo Study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef]
- Raahulan, S.; Sanapalli, B.K.R.; Karri, V.V.S.R. Paclitaxel Loaded Transfersomal Vesicular Drug Delivery for the Treatment of Melanoma Skin Cancers. Int. J. Res. Pharm. Sci. 2019, 10, 2891–2897. [Google Scholar] [CrossRef]
- Gayathri, H.; Sangeetha, S. Pharmaceutical Development of Methotrexate Loaded Transferosomal Gel for Skin Cancer By Doe Approach. J. Pharm. Negat. Results 2022, 13, 2456–2468. [Google Scholar] [CrossRef]
- Gayathri, H.; Sangeetha, S. Design and Development of Tofacitinib Citrate Loaded Transferosomal Gel for Skin Cancer by Box-Behnken Design- Doe Approach. Int. J. Health Sci. 2022, 6, 3119–3140. [Google Scholar] [CrossRef]
- Gayathri, H.; Sangeetha, D.S. Pharmaceutical Development of Tamoxifen Citrate Loaded Transferosomal Gel for Skin Cancer by Doe Approach. J. Posit. Sch. Psychol. 2022, 6, 1879–1890. [Google Scholar]
- Shamim, M.A.; Yeung, S.; Shahid, A.; Chen, M.; Wang, J.; Desai, P.; Parsa, C.; Orlando, R.; Meyskens, F.L., Jr.; Kelly, K.M.; et al. Topical Carvedilol Delivery Prevents UV-Induced Skin Cancer with Negligible Systemic Absorption. Int. J. Pharm. 2022, 611, 121302. [Google Scholar] [CrossRef]
- Jangdey, M.; Kaur, C.; Saraf, S. Efficacy of Concanavalin-A Conjugated Nanotransfersomal Gel of Apigenin for Enhanced Targeted Delivery of UV Induced Skin Malignant Melanoma. Artif. Cells. Nanomed. Biotechnol. 2019, 47, 904–916. [Google Scholar] [CrossRef]
- Deka, T.; Das, M.K.; Das, S.; Das, P.; Singha, L.R. Box-Behnken design approach to develop nano-vesicular herbal gel for the management of skin cancer in experimental animal model. Int. J. Appl. Pharm. 2022, 14, 148–166. [Google Scholar] [CrossRef]
- El-Refaie, W.; Elnaggar, Y.; El-Massik, M.; Abdallah, O. Novel Curcumin-Loaded Gel-Core Hyaluosomes with Promising Burn-Wound Healing Potential: Development, in-Vitro Appraisal and in-Vivo Studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular Carriers as Alternative Drug Delivery Systems: Ethosomes in Focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and Evaluation of Ethosomes for Transdermal Delivery of Lamivudine. AAPS PharmSciTech 2007, 8, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Jain, S. Ethogel Topical Formulation for Increasing the Local Bioavailability of 5-Fluorouracil: A Mechanistic Study. Anticancer Drugs 2012, 23, 923–934. [Google Scholar] [CrossRef]
- Gamal, F.A.; Kharshoum, R.M.; Sayed, O.M.; El-Ela, F.I.A.; Salem, H.F. Control of Basal Cell Carcinoma via Positively Charged Ethosomes of Vismodegib: In Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2020, 56, 101556. [Google Scholar] [CrossRef]
- Gamal, A.; Saeed, H.; El-Ela, F.I.A.; Salem, H.F. Improving the Antitumor Activity and Bioavailability of Sonidegib for the Treatment of Skin Cancer. Pharmaceutics 2021, 13, 1560. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.; Gupta, M.K. Itraconazole Loaded Ethosomal Gel System for Efficient Treatment of Skin Cancer. Int. J. Drug Deliv. 2018, 10, 12–19. [Google Scholar]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin Loaded Binary Ethosomes for Management of Skin Cancer by Dermal Application on UV Exposed Mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Aldosari, B.N.; Al-Subaiyel, A.; Alhaddad, A.; Samman, W.A.; Eleraky, N.E.; Elnaggar, M.G.; Barakat, H.; Tawfeek, H.M. Transethosomal Gel for the Topical Delivery of Celecoxib: Formulation and Estimation of Skin Cancer Progression. Pharmaceutics 2022, 15, 22. [Google Scholar] [CrossRef]
- Sahu, B.; Kori, M. Topical Delivery of Emulsomal Gel for the Management of Skin Cancer. Asian J. Pharm. 2022, 16, 378–386. [Google Scholar]
- Shadab, M.; Alhakamy, N.; Aldawsari, H.; Husain, M.; Khan, N.; Alfaleh, M.; Asfour, H.; Riadi, Y.; Bilgrami, A.; Akhter, M. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers 2021, 13, 923. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.; Kanoujia, J.; Arya, M.; Saraf, S.K.; Saraf, S.A. Process Optimization and Photostability of Silymarin Nanostructured Lipid Carriers: Effect on UV-Irradiated Rat Skin and SK-MEL 2 Cell Line. Drug Deliv. Transl. Res. 2016, 6, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, J.H.; Andrade, L.M.; Esteves, N.L.S.; Brito, L.B.; Valadares, M.C.; Oliveira, G.A.R.; Lima, E.M.; Marreto, R.N.; Gratieri, T.; Taveira, S.F. Topotecan-Loaded Lipid Nanoparticles as a Viable Tool for the Topical Treatment of Skin Cancers. J. Pharm. Pharmacol. 2017, 69, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- El-Sheridy, N.A.; El-Moslemany, R.M.; Ramadan, A.A.; Helmy, M.W.; El-Khordagui, L.K. Itraconazole for Topical Treatment of Skin Carcinogenesis: Efficacy Enhancement by Lipid Nanocapsule Formulations. J. Biomed. Nanotechnol. 2022, 18, 97–111. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil Shell-Enriched Solid Lipid Nanoparticles (SLN) for Effective Skin Carcinoma Treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Imran, M.; Nadeem, M.; Jain, D.; Haider, K.; Moshahid Alam Rizvi, M.; Sheikh, A.; Kesharwani, P.; Kumar jain, G.; Jalees Ahmad, F. Formulation and Development of Novel Lipid-Based Combinatorial Advanced Nanoformulation for Effective Treatment of Non-Melanoma Skin Cancer. Int. J. Pharm. 2023, 632, 122580. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical Nanostructured Lipid Carrier Gel of Quercetin and Resveratrol: Formulation, Optimization, in Vitro and Ex Vivo Study for the Treatment of Skin Cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in Vivo Virtue of Dermal Targeted Combinatorial Lipid Nanocolloidal Based Formulation of 5-Fluorouracil and Resveratrol against Skin Cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J.J.C. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vasile, A.; Ignat, M.; Zaltariov, M.F.; Sacarescu, L.; Stoleriu, I.; Draganescu, D.; Dumitras, M.; Ochiuz, L. Development of New Bexarotene-Loaded Mesoporous Silica Systems for Topical Pharmaceutical Formulations. Acta Chim. Slov. 2018, 65, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ghiciuc, C.M.; Strat, A.L.; Ochiuz, L.; Lupusoru, C.E.; Ignat, M.; Vasile, A.; Grigorovici, A.; Stoleriu, I.; Solcan, C. Inhibition of Bcl-2 and Cox-2 Protein Expression after Local Application of a New Carmustine-Loaded Clinoptilolite-Based Delivery System in a Chemically Induced Skin Cancer Model in Mice. Molecules 2017, 22, 2014. [Google Scholar] [CrossRef]
- Nasr, S.; Rady, M.; Gomaa, I.; Syrovets, T.; Simmet, T.; Fayad, W.; Abdel-Kader, M. Ethosomes and Lipid-Coated Chitosan Nanocarriers for Skin Delivery of a Chlorophyll Derivative: A Potential Treatment of Squamous Cell Carcinoma by Photodynamic Therapy. Int. J. Pharm. 2019, 568, 118528. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Das, P.J.; Pal, P.; Mazumder, B. Topical Delivery of Paclitaxel for Treatment of Skin Cancer. Drug Dev. Ind. Pharm. 2016, 42, 1482–1494. [Google Scholar] [CrossRef]
- Meng, Z.; Fang, X.; Fu, B.; Qian, C.; Yang, Z.; Bai, Y.; Tao, X.; Huang, H.; Ma, C.; Miao, W.; et al. Tumor Immunotherapy Boosted by R837 Nanocrystals through Combining Chemotherapy and Mild Hyperthermia. J. Control. Release 2022, 350, 841–856. [Google Scholar] [CrossRef]
- Boakye, C.H.; Patel, K.; Doddapaneni, R.; Bagde, A.; Behl, G.; Chowdhury, N.; Safe, S.; Singh, M. Ultra-Flexible Nanocarriers for Enhanced Topical Delivery of a Highly Lipophilic Antioxidative Molecule for Skin Cancer Chemoprevention. Colloids Surf. B Biointerfaces 2016, 143, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.M.; Lima, A.C.; Mano, J.F.; Concheiro, A.; Alvarez-Lorenzo, C. Pectin-Coated Chitosan Microgels Crosslinked on Superhydrophobic Surfaces for 5-Fluorouracil Encapsulation. Carbohydr. Polym. 2013, 98, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Zulfakar, M.H. Novel Fish Oil-Based Bigel System for Controlled Drug Delivery and Its Influence on Immunomodulatory Activity of Imiquimod Against Skin Cancer. Pharm. Res. 2016, 34, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Rasti, F.; Yousefpoor, Y.; Abdollahi, A.; Safari, M.; Roozitalab, G.; Osanloo, M. Antioxidative, Anticancer, and Antibacterial Activities of a Nanogel Containing Mentha Spicata L. Essential Oil and Electrospun Nanofibers of Polycaprolactone-Hydroxypropyl Methylcellulose. BMC Complement. Med. Ther. 2022, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction; Prentice Hall: Hoboken, NJ, USA, 2001; ISBN 978-0-201-61091-8. [Google Scholar]
- Seeck, O.H.; Murphy, B. (Eds.) X-Ray Diffraction: Modern Experimental Techniques; Jenny Stanford Publishing: New York, NY, USA, 2015; ISBN 978-0-429-07189-8. [Google Scholar]
- Bacani, R. 7—Gel Characterization: From Molecules to Nanostructure to Macroproperties. In Nano Design for Smart Gels; Bacani, R., Trindade, F., Politi, M.J., Triboni, E.R., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–206. ISBN 978-0-12-814825-9. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 3rd ed.; Brooks Cole: Fort Worth, TX, USA, 2000; ISBN 978-0-03-031961-7. [Google Scholar]
- Hisaki, I.; Shigemitsu, H.; Sakamoto, Y.; Hasegawa, Y.; Okajima, Y.; Nakano, K.; Tohnai, N.; Miyata, M. Octadehydrodibenzo[12]Annulene-Based Organogels: Two Methyl Ester Groups Prevent Crystallization and Promote Gelation. Angew. Chem. Int. Ed. 2009, 48, 5465–5469. [Google Scholar] [CrossRef] [PubMed]
- Voycheva, C.; Slavkova, M.; Popova, T.; Tzankova, D.; Tosheva, A.; Aluani, D.; Tzankova, V.; Ivanova, I.; Tzankov, S.; Spassova, I.; et al. Synthesis and Characterization of PnVCL Grafted Agar with Potential Temperature-Sensitive Delivery of Doxorubicin. J. Drug Deliv. Sci. Technol. 2022, 76, 103725. [Google Scholar] [CrossRef]
- Günther, H. (Ed.) NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ha, W.; Yu, J.; Song, X.; Chen, J.; Shi, Y. Tunable Temperature-Responsive Supramolecular Hydrogels Formed by Prodrugs as a Codelivery System. ACS Appl. Mater. Interfaces 2014, 6, 10623–10630. [Google Scholar] [CrossRef] [PubMed]
- Makhmalzadeh, B.S.; Molavi, O.; Vakili, M.R.; Zhang, H.-F.; Solimani, A.; Abyaneh, H.S.; Loebenberg, R.; Lai, R.; Lavasanifar, A. Functionalized Caprolactone-Polyethylene Glycol Based Thermo-Responsive Hydrogels of Silibinin for the Treatment of Malignant Melanoma. J. Pharm. Pharm. Sci. 2018, 21, 143–159. [Google Scholar] [CrossRef]
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Razak, S.I.A.; Javed, A.; Kadir, M.R.A. Nanocomposite Hydrogels for Melanoma Skin Cancer Care and Treatment: In-Vitro Drug Delivery, Drug Release Kinetics and Anti-Cancer Activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C.A. A Technical Introduction to Transmission Electron Microscopy for Soft-Matter: Imaging, Possibilities, Choices, and Technical Developments. Small 2020, 16, 1906198. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamazaki, M.; Kobiki, Y. Direct Observation of Polymer Gel Surfaces by Atomic Force Microscopy. J. Chem. Phys. 1996, 104, 1751–1757. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure-Function Correlation. Chem. Weinh. Bergstr. Ger. 2018, 24, 762–776. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W.; et al. Xanthan Gum-Based Hydrogel Containing Nanocapsules for Cutaneous Diphenyl Diselenide Delivery in Melanoma Therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef]
- Rosseto, H.C.; de Toledo, L.d.A.S.; dos Santos, R.S.; de Francisco, L.M.B.; Vecchi, C.F.; Esposito, E.; Cortesi, R.; Bruschi, M.L. Design of Propolis-Loaded Film Forming Systems for Topical Administration: The Effect of Acrylic Acid Derivative Polymers. J. Mol. Liq. 2021, 322, 114514. [Google Scholar] [CrossRef]
- Oun, R.; Plumb, J.A.; Wheate, N.J. A Cisplatin Slow-Release Hydrogel Drug Delivery System Based on a Formulation of the Macrocycle Cucurbit[7]Uril, Gelatin and Polyvinyl Alcohol. J. Inorg. Biochem. 2014, 134, 100–105. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial Lipid-Nanosystem for Dermal Delivery of 5-Fluorouracil and Resveratrol against Skin Cancer: Delineation of Improved Dermatokinetics and Epidermal Drug Deposition Enhancement Analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The Influence of the Crystallinity of Lipid Nanoparticles on Their Occlusive Properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The Use of Skin Models in Drug Development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Iqubal, M.K.; Sultana, Y. Tailoring of Berberine Loaded Transniosomes for the Management of Skin Cancer in Mice. J. Drug Deliv. Sci. Technol. 2020, 60, 102051. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Herkenne, C.; Alberti, I.; Naik, A.; Kalia, Y.N.; Mathy, F.-X.; Préat, V.; Guy, R.H. In Vivo Methods for the Assessment of Topical Drug Bioavailability. Pharm. Res. 2008, 25, 87. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The Tape Stripping Procedure—Evaluation of Some Critical Parameters. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Denzer, B.R.; Kulchar, R.J.; Huang, R.B.; Patterson, J. Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels 2021, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Azeera, M.; Vaidevi, S.; Ruckmani, K. Characterization Techniques of Hydrogel and Its Applications. In Cellulose-Based SuperAbsorbent Hydrogels; Mondal, M.D.I.H., Ed.; Polymers and Polymeric Composites: A Reference Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–24. ISBN 978-3-319-76573-0. [Google Scholar]
- Dragicevic-Curic, N.; Winter, S.; Stupar, M.; Milic, J.; Krajišnik, D.; Gitter, B.; Fahr, A. Temoporfin-Loaded Liposomal Gels: Viscoelastic Properties and in Vitro Skin Penetration. Int. J. Pharm. 2009, 373, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.A.; Shehata, T.M.; Mohamed, D.I.; Elsewedy, H.S.; Soliman, W.E. Quality by Design for Development, Optimization and Characterization of Brucine Ethosomal Gel for Skin Cancer Delivery. Molecules 2021, 26, 3454. [Google Scholar] [CrossRef]
- Das, T.; Sengupta, S.; Pal, A.; Sardar, S.; Sahu, N.; Lenka, N.; Panigrahi, K.C.S.; Goswami, L.; Bandyopadhyay, A. Aquasorbent Guargum Grafted Hyperbranched Poly (Acrylic Acid): A Potential Culture Medium for Microbes and Plant Tissues. Carbohydr. Polym. 2019, 222, 114983. [Google Scholar] [CrossRef]
- Zhai, H.; Maibach, H.I. Effects of Skin Occlusion on Percutaneous Absorption: An Overview. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as Versatile and Flexible Nano-Vesicular Carriers in Skin Cancer Therapy: The State of the Art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Geisse, J.K. Medical Therapies for Non-Melanoma Skin Cancer. Clin. Dermatol. 2004, 22, 183–188. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Vaghela, B.; Kayastha, R.; Bhatt, N.; Pathak, N.; Rathod, D. Development and Validation of Dissolution Procedures. J. Appl. Pharm. Sci. 2011, 1, 50–56. [Google Scholar]
- Supe, S.; Takudage, P. Methods for Evaluating Penetration of Drug into the Skin: A Review. Skin Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.d.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of Skin Absorption of Drugs from Topical and Transdermal Formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Schaefer, U.F.; Hansen, S.; Schneider, M.; Contreras, J.L.; Lehr, C.-M. Models for Skin Absorption and Skin Toxicity Testing; Ehrhardt, C., Kim, K.-J., Eds.; Springer: Boston, MA, USA, 2008; Volume VII, pp. 3–33. [Google Scholar]
- Finnin, B.; Walters, K.A.; Franz, T.J. In Vitro Skin Permeation Methodology. In Topical and Transdermal Drug Delivery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 85–108. ISBN 978-1-118-14050-5. [Google Scholar]
- Stagnoli, S.; Garro, C.; Ertekin, O.; Heid, S.; Seyferth, S.; Soria, G.; Correa, N.M.; Leal-Egaña, A.; Boccaccini, A.R. Topical Systems for the Controlled Release of Antineoplastic Drugs: Oxidized Alginate-Gelatin Hydrogel/Unilamellar Vesicles. J. Colloid Interface Sci. 2023, 629, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive Skin Penetration Enhancement and Its Quantification in Vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin Models for the Testing of Transdermal Drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In Vitro Skin Models as a Tool in Optimization of Drug Formulation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed]









| Composition | Additives | API | Particle Size [nm] | Specific Features and/or Comments | Ref. |
|---|---|---|---|---|---|
| PLGA 1 (0.1%), chitosan (0.4%), Poloxamer 108 and 407, PVA 2 (0.4%) | Eucalyptus oil coating | 5-fluorouracil | 190–220 | - | [38] |
| Chitin | - | 5-fluorouracil | 125–140 | - | [124,125] |
| Cross-linked chitosan Poloxamer 407 | Bleomycin | 140–170 | pH sensitive | [126] | |
| Chitosan, TPP 3, Poloxamer 407 | Transcutol® coating | Capecitabine | 120–160 | pH sensitive | [123] |
| Chitosan, TPP 3, Poloxamer 407 | Transcutol® coating | Temozolomide | 170–200 | pH-sensitive | [43] |
| Deacetylated-β-chitosan grafted with ρ-coumaric acid | - | Syzygium aromaticum essential oil | 200–460 | Newly extracted squid β | [127] |
| CMC 4-casein | Casein and folic acid coating | Curcumin | - | Layer by layer coating; folic acid active targeting | [14] |
| Type of Gel | Gelling Agent | API | Particle Size [nm] | Specific Features and/or Comments | Ref. |
|---|---|---|---|---|---|
| Microgel | Chitosan coated with pectin | 5-fluorouracil | 200–600 nm | pH-sensitive | [174] |
| Bigel | Carbopol® 940 (3%) and beeswax (10%) | Imiquimod | - | Hydrogel + oleogel based on fish oil mixed at 50:50 ratio | [175] |
| Nanoemulgel | Poloxamer 407 (20%) | Chrysin | 157 nm | Self-nanoemulsifying preconcentrate was further dispersed in gel | [17] |
| Nanoemulgel | Protasan™ UP G 213 | Daidzein | 190–210 nm | Nanoemulsion-based gel | [18] |
| Nanoemulgel | CMC (3.5%) | Mentha spicata essential oil | 189–464 nm | Gelled nanoemulsion | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavkova, M.; Tzankov, B.; Popova, T.; Voycheva, C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels 2023, 9, 352. https://doi.org/10.3390/gels9050352
Slavkova M, Tzankov B, Popova T, Voycheva C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels. 2023; 9(5):352. https://doi.org/10.3390/gels9050352
Chicago/Turabian StyleSlavkova, Marta, Borislav Tzankov, Teodora Popova, and Christina Voycheva. 2023. "Gel Formulations for Topical Treatment of Skin Cancer: A Review" Gels 9, no. 5: 352. https://doi.org/10.3390/gels9050352
APA StyleSlavkova, M., Tzankov, B., Popova, T., & Voycheva, C. (2023). Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels, 9(5), 352. https://doi.org/10.3390/gels9050352