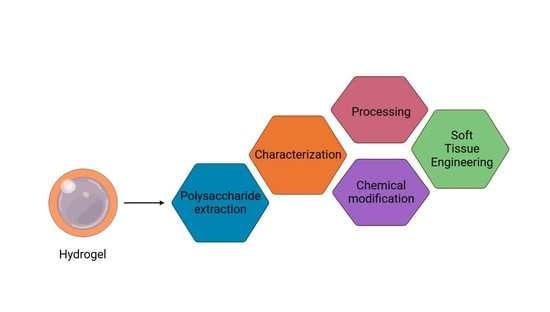
A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering
Abstract
:1. Introduction
2. Natural Biological Substrates
3. Hydrogel Preparation
3.1. Properties of Hydrogel
3.2. Swelling
3.3. Mechanical Properties
3.4. Crosslinking
3.5. Polysaccharides
3.6. Chitosan
3.7. Cellulose
3.8. Characterization
4. Modification of Sodium Alginate
4.1. Physical Blending Modification
4.2. Synthetic Polymer Materials
4.3. Chemical Modification
4.3.1. Oxidation
4.3.2. Sulfation
4.3.3. Graft Copolymerization
4.4. Double-Network SA Hydrogels
4.4.1. Polyvinyl Alcohol Hydrogels
4.4.2. Alginate Hydrogel
4.4.3. Protein-Based Hydrogels
5. Characterization of Sodium-Alginate-Based Hydrogels
5.1. NMR Spectroscopy
5.2. Advantages of NMR Spectroscopy
5.3. Surface-Enhanced Raman Spectroscopy
5.4. Methodology of Sodium Alginate Hydrogel
6. In Vitro Models of Development
7. Applications
7.1. Nanomaterials
7.2. Tissue Engineering
7.3. The Implementation of Sodium Alginate Hydrogel in Regenerative Medicine
7.4. Skin
7.5. Vascular
7.6. Muscle
7.7. Heart
- The restoration of ischemic myocardium can be assisted by chemicals and cells being transported, cells generating growth factors in a particular location, and new blood vessels being generated by cells, as observed by multiple researchers [222,223]. Sodium alginate, which has been sulfated and possesses a structure similar to that of heparin, can administer diverse growth factors and encourage myocardial angiogenesis, as evidenced by the results of [222]. Myocardial stress and apoptosis become successfully reduced, and unfavorable LV remodeling is limited, employing post-infarction mechanical characteristics and biological signals as design criteria [224,225,226] to inform material development. Sodium alginate and fullerenol are two examples of nanomaterial hydrogels that are employed to reduce cardiac stress and give long-term physiological and mechanical support to damaged heart tissue.
- Heart rate stabilization and cardiac contractility restoration following infarction with the administration of electrical impulses [219]. Myocardial infarction causes changes in behavior such as interruption of the normal heart conduction system due to damage to ion channels and connexins. Hydrogels made from sodium alginate that carry electricity could be used to restore heart contractility by delivering electrical impulses and keeping the heartbeat steady [219]. The regeneration of myocardial vasculature is a crucial aspect of tissue engineering for cardiac tissue regeneration. Prior research has employed hydrogel injection to promote cellular proliferation and differentiation, thereby facilitating the generation of blood vessels [227,228,229].
7.8. Alginate Hydrogel in Biomedical Applications
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, L.; Zhang, X.; Chen, L.; Cai, Q.; Yang, X. Hierarchical and heterogeneous hydrogel system as a promising strategy for diversified interfacial tissue regeneration. Biomater. Sci. 2021, 9, 1547–1573. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering complex tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef]
- Jian, H.; Wang, M.; Wang, S.; Wang, A.; Bai, S. 3D bioprinting for cell culture and tissue fabrication. Bio-Des. Manuf. 2018, 1, 45–61. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent trends in three-dimensional bioinks based on alginate for biomedical applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Jovic, T.; Hawkins, K.; Whitaker, I.S. Candidate bioinks for extrusion 3D bioprinting—A systematic review of the literature. Front. Bioeng. Biotechnol. 2021, 9, 616753. [Google Scholar] [CrossRef]
- Murab, S.; Gupta, A.; Włodarczyk-Biegun, M.K.; Kumar, A.; van Rijn, P.; Whitlock, P.; Han, S.S.; Agrawal, G. Alginate based hydrogel inks for 3D bioprinting of engineered orthopedic tissues. Carbohyd. Polym. 2022, 296, 119964. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-conventional methods for gelation of alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Placht, A.M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regenerat. Med. 2017, 11, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Hurtado, A.; Aljabali, A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement strategies for advanced applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Jin, Y.; Compaan, A.; Bhattacharjee, T.; Huang, Y. Granular gel support-enabled extrusion of three-dimensional alginate and cellular structures. Biofabrication 2016, 8, 025016. [Google Scholar] [CrossRef]
- Fernando, I.S.; Kim, D.; Nah, J.W.; Jeon, Y.J. Advances in functionalizing fucoidans and alginates (bio) polymers by structural modifications: A review. Chem. Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Javed, R.; Shah, L.A.; Sayed, M.; Khan, M.S. Uptake of heavy metal ions from aqueous media by hydrogels and their conversion to nanoparticles for generation of a catalyst system: Two-fold application study. RSC Adv. 2018, 8, 14787–14797. [Google Scholar] [CrossRef]
- Richardson, T.; Barner, S.; Candiello, J.; Kumta, P.N.; Banerjee, I. Capsule stiffness regulates the efficiency of pancreatic differentiation of human embryonic stem cells. Acta Biomater. 2016, 35, 153–165. [Google Scholar] [CrossRef]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng 2014, 16, 29–52. [Google Scholar] [CrossRef]
- Tomaszewski, C.E.; DiLillo, K.M.; Baker, B.M.; Arnold, K.B.; Shikanov, A. Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation. Acta Biomater. 2021, 132, 313–324. [Google Scholar] [CrossRef]
- Laronda, M.M.; Duncan, F.E.; Hornick, J.E.; Xu, M.; Pahnke, J.E.; Whelan, K.A.; Shea, L.D.; Woodruff, T.K. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J. Assist. Reprod. Genet. 2014, 31, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rodríguez, D.; Paisano-Cerón, K.; Valdovinos-Ramírez, I.; Solano-Agama, C.; Ortiz-Plata, A.; Mendoza-Garrido, M.E. Three-dimensional alginate-bead culture of human pituitary adenoma cells. J. Vis. Experim. 2016, 108, e53637. [Google Scholar]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent advances in cellulose-based hydrogels for tissue engineering applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.; de Azeredo, H.M.; Aouada, F.A.; Feitosa, J.P.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef]
- Aram, E.; Mehdipour-Ataei, S. Carbon-based nanostructured composites for tissue engineering and drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1167–1188. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Jasim, S.A.; Sharma, S.K.; Margiana, R.; Bokov, D.O.; Obaid, M.A.; Hussein, B.A.; Lafta, H.A.; Jasim, S.F.; Mustafa, Y.F. Alginate-based hydrogels and tubes, as biological macromolecule-based platforms for peripheral nerve tissue engineering: A review. Ann. Biomed. Eng. 2022, 50, 628–653. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Jain, P.K.; Kumar, P.; Pegoretti, A.; Bowen, C.R. Smart manufacturing process of carbon-based low-dimensional structures and fiber-reinforced polymer composites for engineering applications. J. Mater. Eng. Perform. 2020, 29, 4162–4186. [Google Scholar] [CrossRef]
- Ramiah, P.; Du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.; Pillay, V. Hydrogel-based bioinks for 3D bioprinting in tissue regeneration. Front. Mater. 2020, 7, 76. [Google Scholar] [CrossRef]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohyd. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Basu, B. An overview of hydrogel-based bioinks for 3D bioprinting of soft tissues. J. Indian Inst. Sci. 2019, 99, 405–428. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-derived Bioinks from land and marine sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef]
- Selvan, N.K.; Shanmugarajan, T.S.; Uppuluri, V.N.V.A. Hydrogel based scaffolding polymeric biomaterials: Approaches towards skin tissue regeneration. J. Drug Deliv. Sci. Technol. 2020, 55, 101456. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and application as delivery systems of phenolic and aroma compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef]
- Peppas, N.A. Biomedical Applications of Hydrogels Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Lodhi, R.S.; Das, S.; Das, P. Recent Advances in Polymer Hydrogels for Agricultural Applications. In Novel Polymeric Materials for Environmental Applications; World Scientific Publishing Company: Hackensack, NJ, USA, 2023; pp. 109–177. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-based sensor networks: Compositions, properties, and applications—A review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Arndt, K. Hydrogel Sensors and Actuators Volume; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Byju, A.G.; Kulkarni, A.; Gundiah, N. Mechanics of gelatin and elastin-based hydrogels as tissue engineered constructs. In Proceedings of the 13th International Conference Fracture, Beijing, China, 16–21 June 2013; pp. 4406–4415. [Google Scholar]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2013, 59, 44–59. [Google Scholar] [CrossRef]
- Hua, J.; Ng, P.F.; Fei, B. High-strength hydrogels: Microstructure design, characterization, and applications. J. Polym. Sci. 2018, 56, 1325–1335. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. 2009, 90, 720–729. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, D.M.; Chang, W.H.; Huang, R.N.; Hsu, J.C. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: In vitro study. J. Biomed. Mater. Res. 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Gul, K.; Gan, R.Y.; Sun, C.X.; Jiao, G.; Wu, D.T.; Li, H.B.; Kenaan, A.; Corke, H.; Fang, Y.P. Recent advances in the structure, synthesis, and applications of natural polymeric hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 3817–3832. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Omrani, M.; Naimi-Jamal, M.R.; Far, B.F. The design of multi-responsive nanohydrogel networks of chitosan for controlled drug delivery. Carbohyd. Polym. 2022, 298, 120143. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Petrova, V.A.; Romanov, D.P.; Skorik, Y.A. pH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers–Sodium Alginate Polyelectrolyte Complex. Materials 2022, 15, 5860. [Google Scholar] [CrossRef] [PubMed]
- MassanaRoquero, D.; Bollella, P.; Katz, E.; Melman, A. Controlling porosity of calcium alginate hydrogels by interpenetrating polyvinyl alcohol–diboronate polymer network. ACS Appl. Polym. Mater. 2021, 3, 1499–1507. [Google Scholar] [CrossRef]
- Ren, M.; Li, N.; Jiang, X.; Liu, X.; Zou, A. Efficient oral delivery of water-soluble CT contrast agent using an W1/O/W2 alginate hydrogel matrix. Colloids Surf. 2022, 220, 112862. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian-Nodoushan, F.; Nikukar, H.; Soleimani, M.; Jalali-Jahromi, A.; Hosseinzadeh, S.; Khojasteh, A. A smart magnetic hydrogel containing exosome promotes osteogenic commitment of human adipose-derived mesenchymal stem cells. Iran. J. Basic Med. Sci. 2022, 25, 1123. [Google Scholar] [PubMed]
- He, Z.; Luo, H.; Wang, Z.; Chen, D.; Feng, Q.; Cao, X. Injectable and tissue adhesive EGCG-laden hyaluronic acid hydrogel depot for treating oxidative stress and inflammation. Carbohyd. Polym. 2023, 299, 120180. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Singh, S.; Chunglok, W.; Nwabor, O.F.; Chulrik, W.; Jansakun, C.; Bhoopong, P. Porous biodegradable sodium alginate composite fortified with Hibiscus Sabdariffa L. Calyx extract for the multifarious biological applications and extension of climacteric fruit shelf-life. J. Polym. Environ. 2023, 31, 922–938. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. 2020, 8, 10050–10064. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Ma, M.G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-based hydrogels in tissue engineering applications. Cell. Chem. Technol. 2019, 53, 907–923. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Reif, B.; Ashbrook, S.E.; Emsley, L.; Hong, M. Solid-state NMR spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 2. [Google Scholar] [CrossRef]
- Borisov, A.S.; Hazendonk, P.; Hayes, P.G. Solid-state nuclear magnetic resonance spectroscopy: A review of modern techniques and applications for inorganic polymers. J. Inorg. Organomet. Polym. Mater. 2010, 20, 183–212. [Google Scholar] [CrossRef]
- Brown, S.P. Advanced solid-state NMR methods for characterising structure and self-assembly in supramolecular chemistry, polymers and hydrogels. Curr. Opin. Colloid Int. Sci. 2018, 33, 86–98. [Google Scholar] [CrossRef]
- Mathur, A.M.; Scranton, A.B. Characterization of hydrogels using nuclear magnetic resonance spectroscopy. Biomaterials 1996, 17, 547–557. [Google Scholar] [CrossRef]
- Weingarth, M.; Baldus, M. Solid-state NMR-based approaches for supramolecular structure elucidation. Accounts Chem. Res. 2013, 46, 2037–2046. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Sebakhy, K.O. Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomaterials 2021, 11, 1494. [Google Scholar] [CrossRef]
- Polenova, T.; Gupta, R.; Goldbourt, A. Magic angle spinning NMR spectroscopy: A versatile technique for structural and dynamic analysis of solid-phase systems. Anal. Chem. 2015, 87, 5458–5469. [Google Scholar] [CrossRef]
- Ganji, F.; Abdekhodaie, M.J.; Ramazani SA, A. Gelation time and degradation rate of chitosan-based injectable hydrogel. J. Sol-Gel Sci. Technol. 2007, 42, 47–53. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of β-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shen, R.; Yan, Y.; Gao, J. Preparation and characterization of electrospun alginate/PLA nanofibers as tissue engineering material by emulsion eletrospinning. J. Mech. Biomed. Mater. 2017, 65, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Nakamura, M.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Nakagawa, H.; Takiura, K. Development of a three-dimensional bioprinter: Construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J. Biomech. Eng. 2009, 131, 035001. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Luo, G.; Gelinsky, M.; Huang, P.; Ruan, C. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Let. 2009, 189, 295–298. [Google Scholar] [CrossRef]
- Caykara, T.; Demirci, S.; Eroğlu, M.S.; Güven, O. Poly (ethylene oxide) and its blends with sodium alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.I.; et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J. Biomed. Mater. 2006, 78, 1–11. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomat. Sci. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- Daamen, W.F.; Hafmans, T.; Veerkamp, J.H.; Van Kuppevelt, T.H. Comparison of five procedures for the purification of insoluble elastin. Biomaterials 2001, 22, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Zawko, S.A.; Suri, S.; Truong, Q.; Schmidt, C.E. Photopatterned anisotropic swelling of dual-crosslinked hyaluronic acid hydrogels. Acta Biomater. 2009, 5, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Lu, W.W.; Chan, D.; Cheung, K.M.C.; Liu, W.G.; Zhao, F.; Li, Z.Y.; Leong, J.C.Y.; Luk, K.D.K. Osteogenic behavior of alginate encapsulated bone marrow stromal cells: An in vitro study. J. Mater. Sci. 2008, 19, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Arndt, K.F. (Eds.) Hydrogel Sensors and Actuators: Engineering and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Sathaye, S.; Mbi, A.; Sonmez, C.; Chen, Y.; Blair, D.L.; Schneider, J.P.; Pochan, D.J. Rheology of peptide-and protein-based physical hydrogels: Are everyday measurements just scratching the surface. Nanomed. Nanobiotechnol. 2015, 7, 34–68. [Google Scholar] [CrossRef]
- Sahan, A.Z.; Baday, M.; Patel, C.B. Biomimetic Hydrogels in the Study of Cancer Mechanobiology: Overview, Biomedical Applications, and Future Perspectives. Gels 2022, 8, 496. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohyd. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Reakasame, S.; Boccaccini, A.R. Oxidized alginate-based hydrogels for tissue engineering applications: A review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef]
- Gao, C.; Liu, M.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stab. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling alginate oxidation conditions for making alginate-gelatin hydrogels. Carbohyd. Polym. 2018, 198, 509–517. [Google Scholar] [CrossRef]
- Baniasadi, H.; Mashayekhan, S.; Fadaoddini, S.; Haghirsharifzamini, Y. Design, fabrication and characterization of oxidized alginate–gelatin hydrogels for muscle tissue engineering applications. J. Biomater. Appl. 2016, 31, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Barceló, X.; Eichholz, K.F.; Garcia, O.; Kelly, D.J. Tuning the degradation rate of alginate-based bioinks for bioprinting functional cartilage tissue. Biomedicines 2022, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Alioglu, M.A.; Wu, Y.; Ozbolat, V.; Ayan, B.; Dey, M.; Kang, Y.; Ozbolat, I.T. 3D bioprinting of carbohydrazide-modified gelatin into microparticle-suspended oxidized alginate for the fabrication of complex-shaped tissue constructs. ACS Appl. Mater. Int. 2020, 12, 20295–20306. [Google Scholar] [CrossRef]
- Matsumura, K.; Rajan, R. Oxidized polysaccharides as green and sustainable biomaterials. Curr. Org. Chem. 2021, 25, 1483–1496. [Google Scholar] [CrossRef]
- Naghizadeh, Z.; Karkhaneh, A.; Khojasteh, A. Self-crosslinking effect of chitosan and gelatin on alginate-based hydrogels: Injectable in situ forming scaffolds. Mater. Sci. Eng. 2018, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole: PSS hydrogels for tissue engineering. Adv. Healthc. Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Stauber, T.; Levinson, C.; Cavalli, E.; Arlov, Ø.; Zenobi-Wong, M. Tyrosinase-crosslinked, tissue adhesive and biomimetic alginate sulfate hydrogels for cartilage repair. Biomed. Mater. 2020, 15, 045019. [Google Scholar] [CrossRef] [PubMed]
- Malaeb, W.; Bahmad, H.F.; Abou-Kheir, W.; Mhanna, R. The sulfation of biomimetic glycosaminoglycan substrates controls binding of growth factors and subsequent neural and glial cell growth. Biomater. Sci. 2019, 7, 4283–4298. [Google Scholar] [CrossRef]
- Kfoury, G.; El Habbaki, V.; Malaeb, W.; Weaver, S.; Momotenko, D.; Mhanna, R. Alginate sulfate substrates control growth factor binding and growth of primary neurons: Toward engineered 3D neural networks. Adv. Biosyst. 2020, 4, 2000047. [Google Scholar] [CrossRef]
- Freeman, I.; Cohen, S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials 2009, 30, 2122–2131. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, K.; Liu, Z.; Xu, F. Double network hydrogel with high mechanical strength: Performance, progress and future perspective. Sci. China Technol. Sci. 2012, 55, 2241–2254. [Google Scholar] [CrossRef]
- Zhang, M.; Ren, X.; Duan, L.; Gao, G. Joint double-network hydrogels with excellent mechanical performance. Polymer 2018, 153, 607–615. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Bao, H.; Zhao, P.; Jiang, H.; Peng, L.; Yang, X.; Li, C. Solvent-assisted poly (vinyl alcohol) gelated crystalline colloidal array photonic crystals. Soft Matter 2011, 7, 915–921. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Chen, G.; Chu, Z.; Shadike, A.; Chen, C.; Chen, C.; Zhu, Z. Self-healable poly (vinyl alcohol) photonic crystal hydrogel. ACS Appl. Polym. Mater. 2020, 2, 2086–2092. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Negrete-Bolagay, D.; Aza, P.N.D.; Gómez-López, V.M.; López-González, I.; Belén Hernández, A.; De Val, J.E.M.S.; Wu, W. Retinol-Loaded Poly (vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 15623. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and tough hydrogel through physical cross-linking and molecular alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Liu, S.; Zhao, X.; Geng, C.; Wang, L.; Xia, Y. Selected phase separation renders high strength and toughness to polyacrylamide/alginate hydrogels with large-scale cross-linking zones. ACS Appl. Mater. Interfaces 2021, 13, 25383–25391. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohyd. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, T.; Li, S.; Chen, X.; Que, X.; Sheng, L.; Hu, Y.; Peng, J.; Ma, H.; Li, J.; et al. Polyacrylamide/Copper-Alginate Double Network Hydrogel Electrolyte with Excellent Mechanical Properties and Strain-Sensitivity. Macromol. Biosci. 2022, 22, 2100361. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High strength astringent hydrogels using protein as the building block for physically cross-linked multi-network. ACS Appl. Mater. Int. 2017, 10, 7593–7601. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Yamashita, T.; Yatabe, K.; Vogel, V. Mechano-chromic protein–polymer hybrid hydrogel to visualize mechanical strain. Soft Matter 2019, 15, 9388–9393. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, O.; Strand, B.L.; Klepp-Andersen, L.M.; Skjåk-Bræk, G. Analysis of G-block distributions and their impact on gel properties of in vitro epimerized mannuronan. Biomacromolecules 2013, 14, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Murguía-Flores, D.A.; Bonilla-Ríos, J.; Canales-Fiscal, M.R.; Sánchez-Fernández, A. Protein adsorption through Chitosan–Alginate membranes for potential applications. Chem. Central J. 2016, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhu, Y.J.; Li, H.; Zhang, Y.G.; Shen, Y.Q.; Sun, T.W.; Chen, F. Preparation and enhanced mechanical properties of hybrid hydrogels comprising ultralong hydroxyapatite nanowires and sodium alginate. J. Colloid Int. Sci. 2017, 497, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Toti, U.S.; Aminabhavi, T.M. Different viscosity grade sodium alginate and modified sodium alginate membranes in pervaporation separation of water+ acetic acid and water+ isopropanol mixtures. J. Membr. Sci. 2004, 228, 199–208. [Google Scholar] [CrossRef]
- Davis, T.A.; Llanes, F.; Volesky, B.; Diaz-Pulido, G.; McCook, L.; Mucci, A. 1 H-NMR study of Na alginates extracted from Sargassum spp. in relation to metal biosorption. Appl. Biochem. Biotechnol. 2003, 110, 75–90. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Jhon, M.S.; Andrade, J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Böckmann, A.; Gardiennet, C.; Verel, R.; Hunkeler, A.; Loquet, A.; Pintacuda, G.; Emsley, L.; Meier, B.H.; Lesage, A. Characterization of different water pools in solid-state NMR protein samples. J. Biomol. NMR 2009, 45, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; van der Wel, P.C. MAS 1H NMR probes freezing point depression of water and liquid-gel phase transitions in liposomes. Biophys. J. 2016, 111, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of water bound in hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V.; Bogatyrev, V.M.; Zarko, V.I.; Leboda, R.; Goncharuk, E.V.; Novza, A.A.; Turov, A.V.; Chuiko, A.A. Unusual properties of water at hydrophilic/hydrophobic interfaces. Adv. Colloid Int. Sci. 2005, 118, 125–172. [Google Scholar] [CrossRef]
- Gun’Ko, V.M.; Zarko, V.I.; Goncharuk, E.V.; Andriyko, L.S.; Turov, V.V.; Nychiporuk, Y.M.; Leboda, R.; Skubiszewska-Zięba, J.; Gabchak, A.L.; Osovskii, V.D.; et al. TSDC spectroscopy of relaxational and interfacial phenomena. Adv. Colloid Int. Sci. 2007, 131, 1–89. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Habib, M.H.; Alassmy, Y.A.; Abduljawad, M.M.; Alshamrani, K.M.; Sebakhy, K.O. Solid state NMR a powerful technique for investigating sustainable/renewable cellulose-based materials. Polymers 2022, 14, 1049. [Google Scholar] [CrossRef]
- Wang, T.; Hong, M. Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot. 2016, 67, 503–514. [Google Scholar] [CrossRef]
- Munekata, P.E.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural antioxidants from seeds and their application in meat products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Fu, R.; Wang, X.; Li, C.; Santiago-Miranda, A.N.; Pielak, G.J.; Tian, F. In situ structural characterization of a recombinant protein in native Escherichia coli membranes with solid-state magic-angle-spinning NMR. J. Am. Chem. Soc. 2011, 133, 12370–12373. [Google Scholar] [CrossRef]
- Li, B.; Xu, L.; Wu, Q.; Chen, T.; Sun, P.; Jin, Q.; Ding, D.; Wang, X.; Xue, G.; Shi, A.C. Various types of hydrogen bonds, their temperature dependence and water polymer interaction in hydrated poly (acrylic acid) as revealed by 1H solid-state NMR spectroscopy. Macromolecules 2007, 40, 5776–5786. [Google Scholar] [CrossRef]
- van der Wel, P.C. New applications of solid-state NMR in structural biology. Emerg. Topics Life Sci. 2018, 2, 57–67. [Google Scholar]
- Weingarth, M.; Ader, C.; Melquiond, A.S.; Nand, D.; Pongs, O.; Becker, S.; Bonvin, A.M.; Baldus, M. Supramolecular structure of membrane-associated polypeptides by combining solid-state NMR and molecular dynamics simulations. Biophys. J. 2012, 103, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Iggo, J.A.; Adams, D.J. Using solution state NMR spectroscopy to probe NMR invisible gelators. Soft Matter 2015, 11, 7739–7747. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.; Siddiqui, T.; Ali, S.; Farooq, I.; Zafar, M.S.; Khurshid, Z. Nuclear magnetic resonance spectroscopy for medical and dental applications: A comprehensive review. Eur. J. Dent. 2019, 13, 124–128. [Google Scholar] [CrossRef]
- Jiang, J.; Cui, H.; Cao, Y. Preparation and property studies of Zn 3 P 2/calcium alginate. Nano 2014, 9, 1450014. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohyd. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T.; Amado, A.M.; Ribeiro-Claro, P.J. A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009, 21, 599–605. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Chemometric prediction of alginate monomer composition: A comparative spectroscopic study using IR, Raman, NIR and NMR. Carbohyd. Polym. 2008, 72, 730–739. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wagner, M.; Horn, H.; Niessner, R.; Haisch, C. Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal. Bioanalyt. Chem. 2009, 393, 197–206. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Xie, H.; Becraft, E.J.; Baughman, R.H.; Dalton, A.B.; Dieckmann, G.R. Ranking the affinity of aromatic residues for carbon nanotubes by using designed surfactant peptides. J. Pept. Sci. 2008, 14, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Messmer, A.; Yeo, B.S.; Zhang, W.; Zenobi, R. Towards chemical analysis of nanostructures in biofilms II: Tip-enhanced Raman spectroscopy of alginates. Anal. Bioanalyt. Chem. 2008, 391, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.D.T.; Mackie, W.; Parker, K.D.; Smolko, E.E. Crystalline structures of poly-D-mannuronic and poly-L-guluronic acids. J. Polym. Sci. 1971, 9, 311–316. [Google Scholar] [CrossRef]
- Atkins, E.D.T.; Mackie, W.; Smolko, E.E. Crystalline structures of alginic acids. Nature 1970, 225, 626–628. [Google Scholar] [CrossRef]
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117, 1388–1396. [Google Scholar] [CrossRef]
- Gilbert, S.F. Ecological developmental biology: Developmental biology meets the real world. Dev. Biol. 2001, 233, 1–12. [Google Scholar] [CrossRef]
- Krentz, K.J.; Nebel, R.L.; Canseco, R.S.; McGilliard, M.L. In vitro and in vivo development of mouse morulae encapsulated in 2% sodium alginate or 0.1% poly-l-lysine. Theriogenology 1993, 39, 655–667. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Shin, C.S.; Kwak, B.; Han, B.; Park, K. Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug. Mol. Pharm. 2013, 10, 2167–2175. [Google Scholar] [CrossRef]
- Patel, H.R.; Patel, R.R.; Patel, L.D.; Patel, Y.; Raval, A. Preparation and in vitro characterization of non-effervescent floating delivery system of cefpodoxime proxetil. Pharmacophore 2016, 7, 132–140. [Google Scholar]
- Al-Hatamleh, M.A.; Alshaer, W.; Hatmal, M.M.M.; Lambuk, L.; Ahmed, N.; Mustafa, M.Z.; Low, S.C.; Jaafar, J.; Ferji, K.; Six, J.L.; et al. Applications of alginate-based nanomaterials in enhancing the therapeutic effects of bee products. Front. Mol. Biosci. 2022, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of alginate hydrogels for next-generation articular cartilage regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef] [PubMed]
- Alarcin, E.; Bal-Öztürk, A.; Avci, H.; Ghorbanpoor, H.; Dogan Guzel, F.; Akpek, A.; Yesiltas, G.; Canak-Ipek, T.; Avci-Adali, M. Current strategies for the regeneration of skeletal muscle tissue. Int. J. Mol. Sci. 2021, 22, 5929. [Google Scholar] [CrossRef]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of alginates to modulate their physic-chemical properties and obtain biomaterials with different functional properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Grijalvo, S.; Nieto-Díaz, M.; Maza, R.M.; Eritja, R.; Díaz, D.D. Alginate hydrogels as scaffolds and delivery systems to repair the damaged spinal cord. Biotechnol. J. 2019, 14, 1900275. [Google Scholar] [CrossRef]
- Rasool, B.K.A.; FAHMY, S. Development of coated beads for oral controlled delivery of cefaclor: In vitro evaluation. Acta Pharm. 2013, 63, 31–44. [Google Scholar]
- Martinez, A.; Muniz, E.; Iglesias, I.; Teijon, J.M.; Blanco, M.D. Enhanced preclinical efficacy of tamoxifen developed as alginate–cysteine/disulfide bond reduced albumin nanoparticles. Int. J. Pharm. 2012, 436, 574–581. [Google Scholar] [CrossRef]
- Ab-Rahim, S.; Selvaratnam, L.; Raghavendran, H.R.B.; Kamarul, T. Chondrocyte-alginate constructs with or without TGF-β1 produces superior extracellular matrix expression than monolayer cultures. Mol. Cell. Biochem. 2013, 376, 11–20. [Google Scholar] [CrossRef]
- Hill, E.; Boontheekul, T.; Mooney, D.J. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006, 12, 1295–1304. [Google Scholar] [CrossRef]
- Rowley, J.A.; Mooney, D.J. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.K.; Singh, A.; Kipke, D.R. Alginate composition effects on a neural stem cell–seeded scaffold. Tissue Eng. 2009, 15, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.; Siqueira, É.; De Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Fukushima, T.; Hayakawa, T.; Nakashima, N.; Inoue, Y.; Takeda, S.; Okamura, K.; Taniguchi, K. Preparation of carbon nanotube-alginate nanocomposite gel for tissue engineering. Dent. Mater. J. 2006, 25, 719–725. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Goh, K.L.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M. Magnesium oxide nanoparticles reinforced electrospun alginate-based nanofibrous scaffolds with improved physical properties. Int. J. Biomater. 2017, 2017, 1391298. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Yildirim, E.D.; Yin, X.; Nair, K.; Sun, W. Fabrication, characterization, and biocompatibility of single-walled carbon nanotube-reinforced alginate composite scaffolds manufactured using freeform fabrication technique. J. Biomed. Mater. Res. 2008, 87, 406–414. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.Y.W.; Wenger, A.C.; Tam, K.C.; Tang, X.S. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid printing using cellulose nanocrystals reinforced GelMA/HAMA hydrogels for improved structural integration. Adv. Healthc. Mater. 2020, 9, 2001410. [Google Scholar] [CrossRef]
- Temirel, M.; Hawxhurst, C.; Tasoglu, S. Shape fidelity of 3D-bioprinted biodegradable patches. Micromachines 2021, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hagg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Zhao, Y.; Jin, X. Preparation, and characterization of nanoparticle reinforced alginate fibers with high porosity for potential wound dressing application. RSC Adv. 2017, 7, 39349–39358. [Google Scholar] [CrossRef]
- Seal, B.L.; Otero, T.C.; Panitch, A.J.M.S. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. 2019, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate-based hydrogels in soft tissue engineering: A review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef]
- Bushkalova, R.; Farno, M.; Tenailleau, C.; Duployer, B.; Cussac, D.; Parini, A.; Sallerin, B.; Fullana, S.G. Alginate-chitosan PEC scaffolds: A useful tool for soft tissues cell therapy. Int. J. Pharm. 2019, 571, 118692. [Google Scholar] [CrossRef]
- Dahlmann, J.; Krause, A.; Möller, L.; Kensah, G.; Möwes, M.; Diekmann, A.; Martin, U.; Kirschning, A.; Gruh, I.; Dräger, G. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials 2013, 34, 940–951. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust alginate/hyaluronic acid thiol–yne click-hydrogel scaffolds with superior mechanical performance and stability for load-bearing soft tissue engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Liu, Y.; Zhang, C.J.; Zhang, C.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behavior of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Lee, J.S.; Chun, W.; Kim, G.H. An innovative collagen-based cell-printing method for obtaining human adipose stem cell-laden structures consisting of core–sheath structures for tissue engineering. Biomacromolecules 2016, 17, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A new approach for fabricating collagen/ECM-based bio-inks using pre-osteoblasts and human adipose stem cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef]
- Solovieva, E.V.; Fedotov, A.Y.; Mamonov, V.E.; Komlev, V.S.; Panteleyev, A.A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2018, 13, 025007. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Aggor, F.S.; Awad, A.M.; El-Aref, A.T. An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel. Carbohyd. Polym. 2013, 91, 693–698. [Google Scholar] [CrossRef]
- Candiello, J.; Singh, S.S.; Task, K.; Kumta, P.N.; Banerjee, I. Early differentiation patterning of mouse embryonic stem cells in response to variations in alginate substrate stiffness. J. Biol. Eng. 2013, 7, 1–14. [Google Scholar] [CrossRef]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef]
- Kreeger, P.K.; Deck, J.W.; Woodruff, T.K.; Shea, L.D. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006, 27, 714–723. [Google Scholar] [CrossRef]
- Xu, M.; West-Farrell, E.R.; Stouffer, R.L.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol. Reprod. 2009, 81, 587–594. [Google Scholar] [CrossRef]
- King, S.M.; Quartuccio, S.; Hilliard, T.S.; Inoue, K.; Burdette, J.E. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. J. Vis. Exp. 2011, 52, 2804. [Google Scholar]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Wang, J.; Fu, W.; Zhang, D.; Yu, X.; Li, J.; Wan, C. Evaluation of novel alginate dialdehyde cross-linked chitosan/calcium polyphosphate composite scaffolds for meniscus tissue engineering. Carbohyd. Polym. 2010, 79, 705–710. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, C.; Li, L.; Yu, X.; Wang, X.; Peng, H.; Gu, Z.; Wang, Y. In vitro enzymatic degradation of a biological tissue fixed by alginate dialdehyde. Carbohyd. Polym. 2013, 95, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Mooney, D.J.; Arany, P.R.; Lee, K.; Huebsch, N.; Kim, J. Adipose tissue engineering using injectable, oxidized alginate hydrogels. Tissue Eng. 2012, 18, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, A.K.X.; Ho, C.C.; Fang, M.J.; Kuo, T.Y.; Shie, M.Y. The effects of a 3D-printed magnesium-/strontium-doped calcium silicate scaffold on regulation of bone regeneration via dual-stimulation of the AKT and WNT signaling pathways. Biomater. Adv. 2022, 133, 112660. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef]
- Liu, P.; Shen, H.; Zhi, Y.; Si, J.; Shi, J.; Guo, L.; Shen, S.G. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf. 2019, 181, 1026–1034. [Google Scholar] [CrossRef]
- Cheng, L.; Yao, B.; Hu, T.; Cui, X.; Shu, X.; Tang, S.; Wang, R.; Wang, Y.; Liu, Y.; Song, W.; et al. Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation. Int. J. Biol. Macromol. 2019, 135, 1107–1113. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three-dimensional printing bilayer membrane scaffold promotes wound healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Tian, X.; Luo, S.; Yuan, D.; Xu, S.; Yang, L.; Ma, M.; Ye, C. Agarose composite hydrogel and PVA sacrificial materials for bioprinting large-scale, personalized face-like with nutrient networks. Carbohyd. Polym. 2021, 269, 118222. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Zawani, M.; Zulkiflee, I.; Salleh, A.; Fadilah, N.I.M.; Maarof, M.; Wen, A.P.Y.; Duman, F.; Tabata, Y.; Aziz, I.A.; et al. Cellular interaction of human skin cells towards natural bioink via 3D-bioprinting technologies for chronic wound: A comprehensive review. Int. J. Mol. Sci. 2022, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Z.; Zhang, M.; Song, F.; Feng, C.; Liu, H. Simple and robust 3D bioprinting of full-thickness human skin tissue. Bioengineered 2022, 13, 10090–10100. [Google Scholar] [CrossRef]
- Silva, L.P. Current trends and challenges in biofabrication using biomaterials and nanomaterials: Future perspectives for 3D/4D bioprinting. In 3D and 4D Printing in Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 373–421. [Google Scholar]
- Liu, Y.; Hsu, S.H. Biomaterials and neural regeneration. Neural Regen. Res. 2020, 15, 1243. [Google Scholar]
- Guan, S.; Li, J.; Zhang, K.; Li, J. Environmentally responsive hydrogels for repair of cardiovascular tissue. Heart Fail. Rev. 2021, 26, 1273–1285. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D bioprinting in skeletal muscle tissue engineering. Small 2019, 15, 1805530. [Google Scholar] [CrossRef]
- Lian, Q.; Zhou, L.; Li, X.; Mao, W.; Li, D. Perfusive and osmotic capabilities of 3D printed hollow tube for fabricating large-scaled muscle scaffold. Rapid Prototyp. J. 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Sun, Q.; Silva, E.A.; Wang, A.; Fritton, J.C.; Mooney, D.J.; Schaffler, M.B.; Grossman, P.M.; Rajagopalan, S. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm. Res. 2010, 27, 264–271. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef]
- Cattelan, G.; Guerrero Gerbolés, A.; Foresti, R.; Pramstaller, P.P.; Rossini, A.; Miragoli, M.; Caffarra Malvezzi, C. Alginate formulations: Current developments in the race for hydrogel-based cardiac regeneration. Front. Bioeng. Biotechnol. 2020, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Tous, E.; Purcell, B.; Ifkovits, J.L.; Burdick, J.A. Injectable acellular hydrogels for cardiac repair. J. Cardiovasc. Transl. Res. 2011, 4, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.; Radisic, M.; Vunjak-Novakovic, G. Bioactive scaffolds for engineering vascularized cardiac tissues. Macromol. Biosci. 2010, 10, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Ruvinov, E.; Leor, J.; Cohen, S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011, 32, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 2019, 9, 7403. [Google Scholar] [CrossRef]
- Levit, R.D.; Landázuri, N.; Phelps, E.A.; Brown, M.E.; García, A.J.; Davis, M.E.; Joseph, G.; Long Jr, R.; Safley, S.A.; Suever, J.D.; et al. Cellular encapsulation enhances cardiac repair. J. Am. Heart Assoc. 2013, 2, 000367. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Y.; Chen, W.; Li, J.; Wang, X.; Chen, Y.; Yu, Y.; Shen, Z.; Zhang, Y. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J. Mater. Chem. 2020, 8, 308–315. [Google Scholar] [CrossRef]
- Deng, B.; Shen, L.; Wu, Y.; Shen, Y.; Ding, X.; Lu, S.; Jia, J.; Qian, J.; Ge, J. Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction. J. Biomed. Mater. Res. Part A 2015, 103, 907–918. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Seliktar, D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv. Drug Deliv. Rev. 2016, 96, 31–39. [Google Scholar] [CrossRef]
- Ungerleider, J.L.; Christman, K.L. Concise review: Injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: Translational challenges and progress. Stem Cells Transl. Med. 2014, 3, 1090–1099. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Bræk, G.; Espevik, T. Application of alginate gels in biotechnology and biomedicine. Carbohydr. Eur. 1996, 14, 237–242. [Google Scholar]
- Nayak, A.K.; Hasnain, M.S.; Aminabhavi, T.M. Drug delivery using interpenetrating polymeric networks of natural polymers: A recent update. J. Drug Deliv. Sci. Technol. 2021, 66, 102915. [Google Scholar] [CrossRef]
- Singh, S.; Chunglok, W.; Nwabor, O.F.; Ushir, Y.V.; Singh, S.; Panpipat, W. Hydrophilic biopolymer matrix antibacterial peel-off facial mask functionalized with biogenic nanostructured material for cosmeceutical applications. J. Polym. Environ. 2022, 30, 938–953. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical application, patent repository, clinical trial, and regulatory updates on hydrogel: An extensive review. Gels 2021, 7, 207. [Google Scholar] [CrossRef] [PubMed]





| Hydrogel Source | Additional Components | Synthesis Method | Loaded Drug | Ref |
|---|---|---|---|---|
| Chitosan | — | Formaldehyde crosslinking | DOX/5-FU | [54] |
| SA | Carbon nanotube whiskers | Ionic crosslinking | Metronidazole | [55] |
| SA | Polyvinyl alcohol/benzeneboronic acid | Ionic crosslinking | Proteins | [56] |
| SA | — | Ionic crosslinking | Iohexol | [57] |
| SA | Polyvinyl pyrrolidone | Ionic crosslinking | Exosomes | [58] |
| Hyaluronic acid | Gelatin | UV radiation | Epigallocatechin3-gallate | [59] |
| Solid-State NMR | Solution-State NMR | |
|---|---|---|
| Type of sample | All physical states are possible | Only hydrolyzed gels |
| Sample preparation | Preparation (levels of hydration) is straightforward and manageable | Acid hydrolysis makes the preparation process lengthy |
| Restoration of samples | Yes | No |
| Limitations concerning hydrogels | Low sensitivity and resolution | Resolution depends on the solubility |
| Obtained information | Structure and dynamics of intact hydrogel | Chemical structure and composition |
| S. No | Patent No./Country | Title | Disease/Problem | Details |
|---|---|---|---|---|
| 1 | US10799696B2 United States | Stimulating the nasolacrimal gland with a polymer formulation | Dry eye | The hydrogel formulation (made via a UV crosslinking technique) allows therapeutic electrical stimulation of the lacrimal gland, nasal, or sinus tissue to produce tears and treat dry eyes. |
| 2 | US20200085733A1 United States | Formulations of hypotonic hydrogels for improved delivery of therapeutics to mucosal surfaces | Used for diagnosis, prevention, and treatment by inserting into the vagina or colorectum | A polymeric hydrogel (poloxamers) in water acts as a plug and/or is delivered to a mucosal/epithelial surface for therapeutic, preventative, or diagnostic reasons. |
| 3 | CN105209016B China | Matrix hydrogel polymers for cell transport that are biocompatible | Provides cells with a stable environment that promotes their survival and activity | Hydrogel polymer matrices are biocompatible, bioabsorbable, and release cells at the place of application, allowing for localized and precise distribution. |
| 4 | US20180023049A1 United States | Hydrogel formulations without injection for controlled release | Experimenting with cultured cells | Solutions of synthetic peptide hydrogels with a pH of about 3.5and an osmolality in the isotonic range. |
| 5 | US20200360281A1 United States | An interstitial thermo-responsive hydrogel for the treatment of solid tumor malignancies | Solid tumor intra-tumoral chemotherapy | The injectable thermo-responsive hydrogel formed by crosslinking chitosan and genipin into an interpenetrating scaffold can efficiently integrate chemotherapeutic medicines without compromising the hydrogel’s inherent thermo-responsiveness. |
| 6 | JP6293254B2 Japan | Crosslinked hydrophilic coating on a silicone hydrogel lens | Corneal lenses | Contact lenses with a silicone hydrogel coating and a non-silicone hydrogel that is a crosslinked polymer of one or more crosslinkable components and a crosslinked carboxyl-containing polymer material are known as coated silicone hydrogel contact lenses. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Malviya, R.; Singh, S.; Prajapati, B. A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering. Gels 2023, 9, 430. https://doi.org/10.3390/gels9050430
Sharma R, Malviya R, Singh S, Prajapati B. A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering. Gels. 2023; 9(5):430. https://doi.org/10.3390/gels9050430
Chicago/Turabian StyleSharma, Rishav, Rishabha Malviya, Sudarshan Singh, and Bhupendra Prajapati. 2023. "A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering" Gels 9, no. 5: 430. https://doi.org/10.3390/gels9050430
APA StyleSharma, R., Malviya, R., Singh, S., & Prajapati, B. (2023). A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering. Gels, 9(5), 430. https://doi.org/10.3390/gels9050430