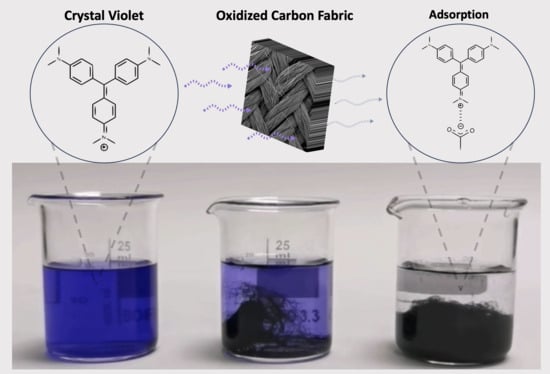
Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbon Fabric Treatment
2.3. Characterization Methods
2.4. Batch Adsorption Experiments
2.5. Adsorption Isotherms
2.6. Thermodynamic Studies
3. Results and Discussion
3.1. Carbon Fabric Characterization
3.2. Adsorption Isotherms
3.3. Adsorption Kinetics
3.4. Effect of the Adsorbent Dosage
3.5. Effect of Temperature on the CV Adsorption
3.6. Thermodynamic Studies
3.7. Adsorption in a Multicomponent System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid. Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Boulahbal, M.; Malouki, M.A.; Canle, M.; Redouane-Salah, Z.; Devanesan, S.; AlSalhi, M.S.; Berkani, M. Removal of the industrial azo dye crystal violet using a natural clay: Characterization, kinetic modeling, and RSM optimization. Chemosphere 2022, 306, 135516. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Li, Q.; Yue, Q.; Sun, H.; Su, Y.; Gao, B.A. Comparative study on the properties, mechanisms and process designs for the adsorption of non-ionic or anionic dyes onto cationic-polymer/bentonite. J. Environ. Manag. 2010, 91, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Omer, A.S.; Naeem, G.A.E.; Elhamid, A.I.A.; Farahat, O.O.M.; El-Bardan, A.A.; Soliman, H.M.A.; Nayl, A.A. Adsorption of crystal violet and methylene blue dyes using a cellulose-based adsorbent from sugercane bagasse: Characterization, kinetic and isotherm studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. In Reviews of Environmental Contamination and Toxicology; de Voogt, W.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; p. 237. [Google Scholar] [CrossRef]
- Akdemir, M.; Isik, B.; Cakar, F.; Cankurtaran, O. Comparison of the adsorption efficiency of cationic (Crystal Violet) and anionic (Congo Red) dyes on Valeriana officinalis roots: Isotherms, kinetics, thermodynamic studies, and error functions. Mater. Chem. Phys. 2022, 291, 126763. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Kumbhar, P.; Narale, D.; Bhosale, R.; Jambhale, C.; Kim, J.; Kolekar, S. Synthesis of tea waste/Fe3O4 magnetic composite (TWMC) for efficient adsorption of crystal violet dye: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2022, 10, 107893. [Google Scholar] [CrossRef]
- Falaki, Z.; Bashiri, H. Preparing an adsorbent from the unused solid waste of Rosewater extraction for high efficient removal of Crystal Violet. J. Iran. Chem. Soc. 2021, 18, 2689–2702. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Sivakumar, V.M.; Thirumarimurugan, M. Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J. Taiwan. Inst. Chem. Eng. 2016, 63, 354–362. [Google Scholar] [CrossRef]
- Mansor, E.S.; Abdallah, H.; Shaban, A.M. Fabrication of high selectivity blend membranes based on poly vinyl alcohol for crystal violet dye removal. J. Environ. Chem. Eng 2020, 8, 103706. [Google Scholar] [CrossRef]
- Pires, I.C.B.; Candido, I.C.M.; Oliveira, H.P. Adsorptive Removal of Crystal Violet from Water by Chemically Modified Coconut Shell. Water Conserv. Sci. Eng. 2020, 5, 159–168. [Google Scholar] [CrossRef]
- Ju, Y.; Fang, J.; Liu, X.; Xu, Z.; Ren, X.; Sun, C.; Yang, S.; Ren, Q.; Ding, Y.; Yu, K.; et al. Photodegradation of crystal violet in TiO 2 suspensions using UV–vis irradiation from two microwave-powered electrodeless discharge lamps (EDL-2): Products, mechanism and feasibility. J. Hazard. Mater. 2011, 185, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Prkait, M.K.; Mohanty, K. Removal of crystal violet by advanced oxidation and microfiltration. Appl. Clay Sci. 2010, 50, 337–341. [Google Scholar] [CrossRef]
- Chen, C.; Kuo, J.; Yang, H.; Chung, Y.A. coupled biological and photocatalysis pretreatment system for the removal of crystal violet from wastewater. Chemosphere 2013, 92, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, A.L.; Nunell, G.V.; Bonelli, P.R. Removal of emerging pollutants from water through adsorption onto carbon-based materials. In Emerging and Nanomaterial Contaminants in Wastewater; Mishra, A.K., Anawar, H.M.D., Drouiche, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 159–213. [Google Scholar]
- Wang, Y.; Peng, C.; Padilla-Ortega, E.; Robledo-Cabrera, A.; López-Valdivieso, A. Cr(VI) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. J. Environ. Chem. Eng. 2020, 8, 104031. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Ioannidis, I.; Pashalidis, I.; Mulla, B.; Kotanidis, G.; Ioannou, K.; Constantinides, G.; Kostoglou, N.; Rebholz, C. Radionuclide Removal from Aqueous Solutions Using Oxidized Carbon Fabrics. Materials 2023, 16, 7429. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.; Hussain, M.A. Review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated Carbon for Dyes Removal: Modeling and Understanding the Adsorption Process. J. Chem. 2020, 2020, 2096834. [Google Scholar] [CrossRef]
- Cukierman, A.L. Development and Environmental Applications of Activated Carbon Cloths. Int. Sch. Res. Not. 2013, 2013, 261523. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon (Origins). In Activated Carbon; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2006; pp. 13–86. [Google Scholar]
- Zulfiqar, U.; Kostoglou, N.; Thomas, A.G.; Rebholz, C.; Matthews, A.; Lewis, D.J. Flexible nanoporous activated carbon for adsorption of organics from industrial effluents. Nanoscale 2021, 13, 15311–15323. [Google Scholar] [CrossRef]
- Kostoglou, N.; Koczwara, C.; Prehal, C.; Terziyska, V.; Babic, B.; Matovic, B.; Constantinides, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; et al. Nanoporous activated carbon cloth as a versatile material for hydrogen adsorption, selective gas separation and electrochemical energy storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Ahsaine, H.A.; Anfar, Z.; Zbair, M.; Ezahri, M.; El Alem, N. Adsorptive Removal of Methylene Blue and Crystal Violet onto Micro-Mesoporous Zr 3 O/Activated Carbon Composite: A Joint Experimental and Statistical Modeling Considerations. J. Chem. 2018, 2018, 6982014. [Google Scholar] [CrossRef]
- Sultana, S.; Islam, K.; Hasan, M.A.; Khan, H.M.J.; Khan, M.A.R.; Deb, A.; Al Raihan, M.; Rahman, M.W. Adsorption of crystal violet dye by coconut husk powder: Isotherm, kinetics and thermodynamics perspectives. Environ. Nanotechnol. Monit. 2022, 17, 100651. [Google Scholar] [CrossRef]
- Porkodi, K.; Kumar, K.V. Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: Eosin yellow, malachite green and crystal violet single component systems. J. Hazard. Mater. 2007, 143, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Sarabadan, M.; Bashiri, H.; Mousavi, S.M. Removal of crystal violet dye by an efficient and low cost adsorbent: Modeling, kinetic, equilibrium and thermodynamic studies. Korean J. Chem. Eng. 2019, 36, 1575–1586. [Google Scholar] [CrossRef]
- Abbas, M.; Harrache, Z.; Trari, M. Removal of gentian violet in aqueous solution by activated carbon equilibrium, kinetics, and thermodynamic study. Adsorp Sci Technol. 2019, 37, 566. [Google Scholar] [CrossRef]
- Gohr, M.S.H.; Abd-Elhamid, A.I.; El-Shanshory, A.A.; Soliman, H.M.A. Adsorption of cationic dyes onto chemically modified activated carbon: Kinetics and thermodynamic study. J. Mol. Liq. 2022, 346, 118227. [Google Scholar] [CrossRef]
- Goswami, R.; Dey, A.K. Use of Anionic Surfactant-Modified Activated Carbon for Efficient Adsorptive Removal of Crystal Violet Dye. Adsorp. Sci. Technol. 2022, 2022, 2357242. [Google Scholar] [CrossRef]
- Duman, O.; Ayranci, E. Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J. Hazard. Mater. 2010, 174, 359–367. [Google Scholar] [CrossRef]
- Kumari, M.; Chaudhary, G.R.; Chaudhary, S.; Umar, A. Transformation of solid plastic waste to activated carbon fibres for wastewater treatment. Chemosphere 2022, 294, 133692. [Google Scholar] [CrossRef]
- ISO 9277:2022—Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Standard Organization. Available online: https://www.iso.org/standard/71014.html (accessed on 19 February 2023).
- Stevens, J.S.; Byard, S.J.; Seaton, C.C.; Sadiq, G.; Davey, R.J.; Schroeder, S.L.M. Proton transfer and hydrogen bonding in the organic solid state: A combined XRD/XPS/ssNMR study of 17 organic acid–base complexes. Phys. Chem. Chem. Phys. 2014, 16, 1150–1160. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef]
- Kumar, C.V.; Pattammattel, A. Chapter 3—Characterization techniques for graphene. In Introduction to Graphene; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–74. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface characterization of oxidized biochar fibers derived from Luffa Cylindrica and lanthanide binding. J. Environ. Chem. Eng. 2017, 5, 4069. [Google Scholar] [CrossRef]
- Mahmud, N.A.; Osman, N.; Jani, A.M.M. Characterization of Acid Treated Activated Carbon From Oil Palm Empty Fruit Bunches (EFB). J. Phys. Conf. Ser. 2018, 1083, 12049. [Google Scholar] [CrossRef]
- Pereira, M.F.R.; Soares, S.F.; Órfão, J.J.M.; Figueiredo, J.L. Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon 2003, 41, 811. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Prodromou, M.; Pashalidis, I. Activated biochar derived from cactus fibres—Preparation, characterization and application on Cu(II) removal from aqueous solutions. Bioresour. Technol. 2014, 159, 460–464. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized biochar obtained from pine needles as a novel adsorbent to remove caffeine from aqueous solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H. High surface area activated carbon derived from chitin for efficient adsorption of Crystal Violet. Diam. Relat. Mater. 2021, 118, 108516. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G.A. Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? J. Mol. Liq. 2016, 218, 174–185. [Google Scholar] [CrossRef]













| Material | SBET (m2/g) | VGurvich (cm3/g) | W (nm) |
|---|---|---|---|
| AC-F | 1080 | 0.49 | 0.90 |
| AC-FO | 475 | 0.25 | 1.05 |
| AC-W | 1119 | 0.47 | 0.84 |
| AC-WO | 560 | 0.29 | 1.03 |
| AC-NW | 1845 | 0.86 | 0.93 |
| AC-NWO | 532 | 0.32 | 1.20 |
| Parameter | AC-F | AC-FO | AC-W | AC-WO | AC-NW | AC-NWO |
|---|---|---|---|---|---|---|
| qm (mg/g) | 34 | 130 | 39 | 114 | 155 | 278 |
| KL (L/mg) | 0.25 | 0.46 | 0.02 | 0.11 | 0.38 | 2.72 |
| R2 | 0.999 | 0.997 | 0.996 | 0.998 | 0.999 | 1.000 |
| Carbon Cloth | Pseudo 1st Order | Pseudo 2nd Order | |||||
|---|---|---|---|---|---|---|---|
| qt (mg/g) | k1 (min−1) | R2 | qt (mg/g) | k2 (min−1) | R2 | qt (Experimental) (mg/g) | |
| AC-F | 0.8 | 0.04 | 0.934 | 1.0 | 0.06 | 0.828 | 0.8 |
| AC-FO | 7.4 | 0.12 | 0.977 | 10.4 | 0.04 | 0.999 | 10.0 |
| AC-W | 0.1 | 0.04 | 0.922 | 0.2 | 0.90 | 0.969 | 0.2 |
| AC-WO | 0.7 | 0.06 | 0.952 | 0.9 | 0.15 | 0.976 | 0.9 |
| AC-NW | 7.5 | 0.05 | 0.952 | 9.8 | 0.01 | 0.969 | 9.1 |
| AC-NWO | 8.5 | 0.13 | 0.941 | 10.5 | 0.03 | 0.996 | 10.0 |
| Parameter | Temperature (K) | |
|---|---|---|
| 318 | 328 | |
| qm (mg/g) | 355 | 428 |
| KL (L/mg) | 0.26 | 0.34 |
| R2 | 0.999 | 0.999 |
| Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol∙K) |
|---|---|---|---|
| 318 | −41.2 | 23.5 | 200 |
| 328 | −43.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulla, B.; Ioannou, K.; Kotanidis, G.; Ioannidis, I.; Constantinides, G.; Baker, M.; Hinder, S.; Mitterer, C.; Pashalidis, I.; Kostoglou, N.; et al. Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics. C 2024, 10, 19. https://doi.org/10.3390/c10010019
Mulla B, Ioannou K, Kotanidis G, Ioannidis I, Constantinides G, Baker M, Hinder S, Mitterer C, Pashalidis I, Kostoglou N, et al. Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics. C. 2024; 10(1):19. https://doi.org/10.3390/c10010019
Chicago/Turabian StyleMulla, Batuhan, Kyriacos Ioannou, Gkerman Kotanidis, Ioannis Ioannidis, Georgios Constantinides, Mark Baker, Steven Hinder, Christian Mitterer, Ioannis Pashalidis, Nikolaos Kostoglou, and et al. 2024. "Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics" C 10, no. 1: 19. https://doi.org/10.3390/c10010019
APA StyleMulla, B., Ioannou, K., Kotanidis, G., Ioannidis, I., Constantinides, G., Baker, M., Hinder, S., Mitterer, C., Pashalidis, I., Kostoglou, N., & Rebholz, C. (2024). Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics. C, 10(1), 19. https://doi.org/10.3390/c10010019