Continuous Reactive-Roll-to-Roll Growth of Carbon Nanotubes for Fog Water Harvesting Applications
Abstract
:1. Introduction
2. Fog Harvester Basics and Requirements
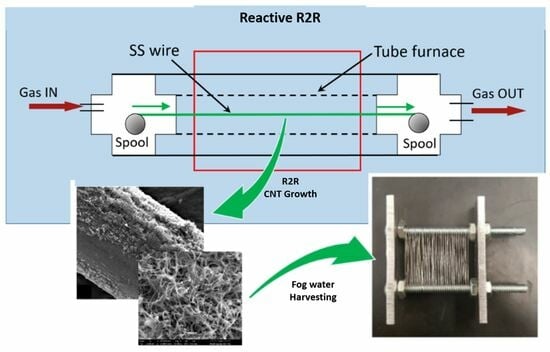
3. Generation of a CNT-on-SS Wire Structure in a Continuous RR2R Process
Methodology for Dynamic RR2R MWCNT Growth
4. Experimental Results
4.1. CNT Static Growth on SS Wires
4.2. CNT Static Growth on SS Grids
4.3. Transition from Static to Moving Wire
5. Fog Harvesting Experiments
5.1. Fog Harvesting Setup
5.2. Fog Harvesting Results
6. Discussion
7. Conclusions
- The direct growth technique uses a material-specific design for generating nanoscale surface roughness patterns for CNT nucleation and growth and provides a CNT forest anchorage with primary bonds with the host surface. In other words, the CNT forest is inherently linked to strong anchorage to the SS surface;
- No external catalyst, dye solution, slurry, or other deposition technique is required; it is the stainless-steel substrate itself that is acting as the CNT growth and anchoring site;
- The simple catalyst-free RR2R technique continuously generates reliably very long CNT-covered wires able to generate the very large collecting surfaces required, together with a CNT forest oriented in a direction perpendicular to the surface;
- The CNT-covered wires intrinsically provide a much larger surface area for atmospheric-suspended micro-droplet collection compared to bare wires. More importantly, both of the required local hydrophilic/hydrophobic properties needed for water capture and drainage are generated;
- Finally, in view of possibly extending the RR2R technique to other applications requiring large surfaces and various geometries, it is to be noted that the Direct-Growth technique based on a relatively simple metallurgical surface transformation allows the possibility of various surface shapes and sizes, as was previously demonstrated by growth generated on particles, flat surfaces, and grids.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdul-Wahab, S.; Lea, V. Reviewing fog water collection worldwide and in Oman. Int. J. Environ. Stud. 2008, 65, 487–500. [Google Scholar] [CrossRef]
- Schemenauer, R.; Fuenzalida, H.; Cereceda, P. A Neglected Water Resource: The Camanchaca of South America. Bull. Am. Meteorol. Soc. 1988, 69, 138–147. [Google Scholar] [CrossRef]
- Jarimi, R.; Powel, R.; Riffat, S. Review of sustainable methods for atmospheric water harvesting. Int. J. Low-Carbon Technol. 2020, 15, 253–276. [Google Scholar] [CrossRef]
- Klemm, O.; Schemenauer, R.S.; Lummerich, A.; Cereceda, P.; Marzol, V.; Corell, D.; Van Heerden, J.; Reinhard, D.; Gherezghiher, T.; Olivier, J.; et al. Fog as a Fresh-Water Ressource: Overview and Perspectives. AMBIO 2012, 41, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Sharma, B.R.; Bruggeman, R.; Choukr-Allah, R.; Karajeh, F. Non-conventional water resources and opportunities for water augmentation to achieve food security in water scarce countries. Agric. Water Manag. 2007, 87, 2–22. [Google Scholar] [CrossRef]
- Schemenauer, R.S.; Cereceda, P. Fog-Water Collection in Arid Coastal Locations. Ambio 1991, 20, 303–308. [Google Scholar]
- Pangarkar, B.; Sane, M.; Guddad, M. Reverse Osmosis and Membrane Distillation for Desalination of Groundwater: A Review. ISRN Mater. Sci. 2011, 2011, 523124. [Google Scholar] [CrossRef]
- Zotalis, K.; Dialynas, E.; Mamassis, N.; Angelakis, A. Desalination technologies: Hellenic experience. Water 2014, 6, 1134–1150. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; Van Vliet, M.; Smakhtin, V.; Kang, S. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Gude, V. Energy consumption and recovery in reverse osmosis. Desalination Water Treat. 2011, 36, 239–260. [Google Scholar] [CrossRef]
- Al-Karaghouli, A.; Kazmerski, L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Greenlee, L.; Lawler, D.; Freeman, B.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Beltran, J.; Koo-Oshima, S. Water Desalinisation for Agricultural Applications; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- WHO. Technical Notes on Drinking-Water, Sanitation and Hygiene in Emergencies. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/who-tn-09-how-much-water-is-needed.pdf?sfvrsn=1e876b2a_6 (accessed on 3 January 2024).
- Gleick, P.H. Water in Crisis—A Guide to the World’s Fresh Water Resources; Oxford University Press: Oxford, UK, 1993; pp. 13–24. [Google Scholar]
- Legrand, U.; Klassen, D.; Watson, S.; Aufoujal, A.; Nisol, B.; Boudreault, R.; Waters, K.; Meunier, J.L.; Girard-Lauriault, P.L.; Wertheimer, M.; et al. Nanoporous sponges as carbon-based sorbents for atmospheric water generation. Ind. Eng. Chem. Res. 2021, 60, 12923–12933. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S. Best practices for solar water production technologies. Nat. Sustain. 2022, 5, 554–556. [Google Scholar] [CrossRef]
- Bai, H.; Wang, L.; Ju, J.; Sun, R.; Zheng, Y.; Jiang, L. Efficient Water Collection on Integrative Bioinspired Surfaces with Star-Shaped Wettability Patterns. Adv. Mater. 2014, 26, 5025–5030. [Google Scholar] [CrossRef]
- Shang, L. Bioinspired Multifunctional Spindle-Knotted Microfibers from Microfluidics. Small 2017, 14, 1600286. [Google Scholar] [CrossRef]
- Yu, Z.; Yun, F.F.; Wang, Y.; Yao, L.; Dou, S.; Liu, K.; Jiang, L.; Wang, X. Desert Beetle-Inspired Superwettable Patterned Surfaces for Water Harvesting. Small 2017, 13, 36. [Google Scholar] [CrossRef]
- Shi, W.; De Koninck, L.H.; Hart, B.J.; Kowalski, N.G.; Fugaro, A.P.; Van der Sloot, T.; Ott, R.S.; Kenedy, B.S.; Boreyko, J.B. Harps under Heavy Fog Conditions: Superior to Meshes but Prone to Tangling. ACS Appl. Mater. Interfaces 2020, 12, 48124–48132. [Google Scholar] [CrossRef]
- Lei, J.; Guo, Z. A fog-collecting surface mimicking the Namib beetle: Its water collection efficiency and influencing factors. Nanoscale 2020, 12, 6921–6936. [Google Scholar] [CrossRef]
- Wen, C.; Guo, H.; Bai, H.; Xu, T.; Liu, M.; Yang, J.; Zhu, Y.; Zhao, W.; Zhang, J.; Zhang, L. Beetle-Inspired Hierarchical Antibacterial Interface for Reliable Fog Harvesting. ACS Appl. Mater. Interfaces 2019, 11, 34330–34337. [Google Scholar] [CrossRef]
- Seo, D.; Lee, J.; Lee, C.; Nam, Y. The effects of surface wettability on the fog and dew moisture harvesting performance on tubular surfaces. Sci. Rep. 2016, 6, 24276. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Ellerbrok, D.; Barthlott, W.; Koch, K. Fog collecting biomimetic surfaces: Influence of microstructure and wettability. Bioinspir. Biomim. 2015, 10, 016004. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Van der Slot, T.; Hart, B.; Kennedy, B.; Boreyko, J. Harps Enable Water Harvesting under Light Fog Conditions. Adv. Sustain. Syst. 2020, 4, 2000040. [Google Scholar] [CrossRef]
- Park, K.; Chhatre, S.; Srinivasan, S.; Cohen, R.; Mckinley, G. Optimal Design of Permeable Fiber Network Structures for Fog Harvesting. Langmuir 2013, 29, 13269–13277. [Google Scholar] [CrossRef]
- Regalado, C.; Ritter, A. The design of an optimal fog water collector: A theoretical analysis. Atmos. Res. 2016, 178–179, 45–54. [Google Scholar] [CrossRef]
- de Dios Rivera, J. Aerodynamic collection efficiency of fog water collectors. Atmos. Res. 2011, 102, 335–342. [Google Scholar] [CrossRef]
- Shi, W.; Anderson, M.; Tulkoff, J.; Kennedy, B.; Boreyko, J. Fog Harvesting with Harps. ACS Appl. Mater. Interfaces 2018, 10, 11979–11986. [Google Scholar] [CrossRef]
- Hordy, N.; Coulombe, S.; Meunier, J.L. Plasma functionalization of carbon nanotubes for the synthesis of stable aqueous nanofluids and poly(vinyl alcohol) nanocomposites. Plasma Process. Polym. 2013, 10, 110–118. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Hordy, N.; Mendoza-Gonzalez, N.Y.; Coulombe, S.; Meunier, J.-L. The effect of carbon input on the morphology and attachment of carbon nanotubes grown directly from stainless steel. Carbon 2013, 63, 348–357. [Google Scholar] [CrossRef]
- Arcila-Velez, M.R.; Zhu, J.; Childress, A.; Karakaya, M.; Podila, R.; Rao, A.M.; Roberts, M.E. Roll-to-roll synthesis of vertically aligned carbon nanotube electrodes for electrical double layer capacitors. Nano Energy 2014, 8, 9–16. [Google Scholar] [CrossRef]
- Rashid, T.; Liang, H.-L.; Taimur, M.; Chiodarelli, N.; Khawaja, H.A.; Edvardsen, K.; de Volder, M. Roll to roll coating of carbon nanotube films for electro thermal heating. Cold Reg. Sci. Technol. 2021, 182, 103210. [Google Scholar] [CrossRef]
- Zhang, S.; Leonhardt, B.E.; Nguyen, N.; Oluwalowo, A.; Jolowsky, C.; Hao, A.; Liang, R.; Park, J.G. Roll-to-roll continuous carbon nanotube sheets with high electrical conductivity. RSC Adv. 2018, 8, 12692–12700. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lim, X.; Zhu, Y.; Yu, T.; Ong, C.K.; Shen, Z.; Wee, A.T.-S.; Sow, C.H. Tailoring Wettability Change on Aligned and Patterned Carbon Nanotube Films for Selective Assembly. J. Phys. Chem. B 2007, 111, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Rodes, C.; Smith, T.; Crouse, R.; Ramachandran, G. Measurements of the Size Distribution of Aerosols Produced by Ultrasonic Humidification. Aerosol Sci. Technol. 1990, 13, 220–229. [Google Scholar] [CrossRef]
- Yule, A.; Al-Suleimani, Y. On droplet formation from capilary waves on a vibrating surface. Math. Phys. Eng. Sci. 1997, 456, 1069–1085. [Google Scholar] [CrossRef]
- Kooij, S.; Astefanei, A.; Corthals, G.; Bonn, D. Size distributions of droplets produced by ultrasonic nebulizers. Sci. Rep. 2019, 9, 6128. [Google Scholar] [CrossRef]
- Ritter, A.; Guerra, C.; Water, J. Quantification of Fog Water Collection in Three Locations of Tenerife (Canary Islands). Water 2015, 7, 3306–3319. [Google Scholar] [CrossRef]
- Pérez-Diaz, J.L.; Ivanov, O.; Peshev, Z.; Alvarez-Valuenzuela, M.A.; Valiente-Blanco, I.; Evgenieva, T.; Dreischuh, T.; Gueorguiev, O.; Todorov, P.V.; Vaseashta, A. Fogs: Physical Basis, Characteristic Properties, and Impacts on the Environment and Human Health. Water 2017, 9, 807. [Google Scholar] [CrossRef]
- Luo, C.; Wang, X. Conditions for Barrel and Clam-Shell Liquid Drops to Move on Bio-inspired Conical Wires. Sci. Rep. 2017, 7, 9717. [Google Scholar] [CrossRef]
- Schemenauer, R.; Joe, P. The collection efficiency of a massive fog collector. Atmos. Res. 1989, 24, 53–69. [Google Scholar] [CrossRef]
- Pajootan, E.; Amin, S.; Omanovic, S.; Coulombe, S. Radio frenquency plasma-assisted pulsed laser deposited Pt/TiOxNy coatings on multiwalled carbon nanotubes as gas diffusion electrodes for the oxygen reduction reaction. Adv. Mater. Technol. June 2022, 7, 2200196. [Google Scholar] [CrossRef]
- Hordy, N.; Rabilloud, D.; Coulombe, S.; Meunier, J.L. A stable carbon nanotube nanofluid for latent heat-driven volumetric absorption solar heating applications. J. Nanomater. 2015, 2015, 850217. [Google Scholar] [CrossRef]
- Rao, L.; Reddy, N.; Coulombe, S.; Meunier, J.; Munz, R. Carbon nanotubes as nanoparticle collector. J. Nanoparticle Res. 2007, 9, 689–695. [Google Scholar] [CrossRef]
- Sridhar, D.; Meunier, J.L.; Omanovic, S. Directly grown carbon nano-fibers on nickel foam as binder-free long lasting supercapacitor electrodes. Mater. Chem. Phys. 2019, 223, 434–440. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meunier, J.-L.; Ouellet, J.; Basu, K.; Aufoujal, A.; Boudreault, R.; Tavares, J.R. Continuous Reactive-Roll-to-Roll Growth of Carbon Nanotubes for Fog Water Harvesting Applications. C 2024, 10, 9. https://doi.org/10.3390/c10010009
Meunier J-L, Ouellet J, Basu K, Aufoujal A, Boudreault R, Tavares JR. Continuous Reactive-Roll-to-Roll Growth of Carbon Nanotubes for Fog Water Harvesting Applications. C. 2024; 10(1):9. https://doi.org/10.3390/c10010009
Chicago/Turabian StyleMeunier, Jean-Luc, Jeanne Ouellet, Kaustubh Basu, Alessio Aufoujal, Richard Boudreault, and Jason Robert Tavares. 2024. "Continuous Reactive-Roll-to-Roll Growth of Carbon Nanotubes for Fog Water Harvesting Applications" C 10, no. 1: 9. https://doi.org/10.3390/c10010009
APA StyleMeunier, J. -L., Ouellet, J., Basu, K., Aufoujal, A., Boudreault, R., & Tavares, J. R. (2024). Continuous Reactive-Roll-to-Roll Growth of Carbon Nanotubes for Fog Water Harvesting Applications. C, 10(1), 9. https://doi.org/10.3390/c10010009