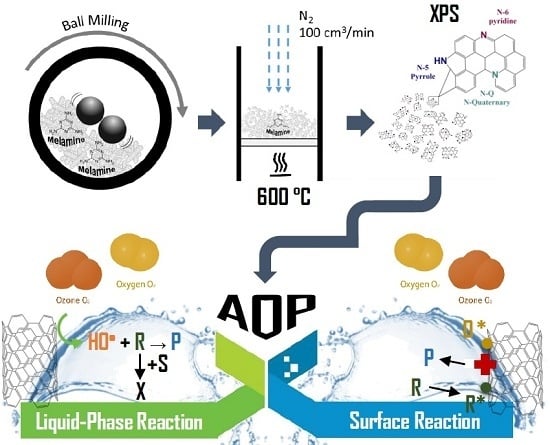
Tuning CNT Properties for Metal-Free Environmental Catalytic Applications
Abstract
:1. Introduction
2. Tuning Carbon Nanotube Properties
2.1. Oxygen-Containing Surface Groups
2.2. Nitrogen-Containing Surface Groups
2.3. Sulfonic Acid Surface Groups
3. Environmental Catalytic Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AOPs | Advanced oxidation processes |
| CNT | Carbon Nanotube |
| COZ | Catalytic Ozonation |
| CWAO | Catalytic Wet Air Oxiation |
| EELS | Electron Energy Loss Spectroscopy |
| HT | Hydrothermal |
| IR | Infrared Spectroscopie |
| N- | Nitrogen |
| N-5 | Pyrrolic-like group |
| N-6 | Pyridinic-like group |
| N-Q | Graphitic-like group |
| O- | Oxygen |
| pHpzc | Point of Zero Charge |
| S- | Sulfur |
| STM | Scanning Tunneling Microscope |
| TEM | Transmission Electron Microscopy |
| TG | Thermogravimetry |
| TPD | Temperature Programmed Desorption |
| WAO | Wet Air Oxidation |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ovejero, G.; Sotelo, J.L.; Romero, M.D.; Rodríguez, A.; Ocaña, M.A.; Rodríguez, G.; García, J. Multiwalled carbon nanotubes for liquid-phase oxidation. Functionalization, characterization, and catalytic activity. Ind. Eng. Chem. Res. 2006, 45, 2206–2212. [Google Scholar] [CrossRef]
- Paradise, M.; Goswami, T. Carbon nanotubes—Production and industrial applications. Mater. Des. 2007, 28, 1477–1489. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sun, X.; Wang, R.; Su, D. Research progress in metal-free carbon-based catalysts. Chin. J. Catal. 2013, 34, 508–523. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. Carbon as catalyst. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 177–217. [Google Scholar]
- Reiche, S.; Blume, R.; Zhao, X.C.; Su, D.; Kunkes, E.; Behrens, M.; Schlögl, R. Reactivity of mesoporous carbon against water—An in-situ XPS study. Carbon 2014, 77, 175–183. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Boehm, H.-P. Catalytic properties of nitrogen-containing carbons. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Figueiredo, J.L.; Silva, A.M.T.; Faria, J.L. The role of activated carbons functionalized with thiol and sulfonic acid groups in catalytic wet peroxide oxidation. Appl. Catal. B Environ. 2011, 106, 390–397. [Google Scholar] [CrossRef]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Silva, A.M.T.; Faria, J.L. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef]
- Terzyk, A.P. Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. J. Colloid Interface Sci. 2003, 268, 301–329. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Howard Fairbrother, D. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Álvarez-Méndez, J.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal. Chim. Acta 2015, 853, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, H.S.; Kim, Y.J.; Chung, H. Structural modification of carbon nanotubes by various ball milling. J. Alloy. Compd. 2007, 434, 428–432. [Google Scholar] [CrossRef]
- Tucho, W.M.; Mauroy, H.; Walmsley, J.C.; Deledda, S.; Holmestad, R.; Hauback, B.C. The effects of ball milling intensity on morphology of multiwall carbon nanotubes. Scr. Mater. 2010, 63, 637–640. [Google Scholar] [CrossRef]
- Pierard, N.; Fonseca, A.; Colomer, J.F.; Bossuot, C.; Benoit, J.M.; Van Tendeloo, G.; Pirard, J.P.; Nagy, J.B. Ball milling effect on the structure of single-wall carbon nanotubes. Carbon 2004, 42, 1691–1697. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Gonçalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of carbon nanotubes by ball-milling to be used as ozonation catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts. Carbon 2010, 48, 4369–4381. [Google Scholar] [CrossRef]
- Byl, O.; Liu, J.; Yates, J.T. Etching of carbon nanotubes by ozonea surface area study. Langmuir 2005, 21, 4200–4204. [Google Scholar] [CrossRef] [PubMed]
- Gerber, I.; Oubenali, M.; Bacsa, R.; Durand, J.; Gonçalves, A.; Pereira, M.F.R.; Jolibois, F.; Perrin, L.; Poteau, R.; Serp, P. Theoretical and experimental studies on the carbon-nanotube surface oxidation by nitric acid: Interplay between functionalization and vacancy enlargement. Chem. Eur. J. 2011, 17, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for mwcnt functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Rocha, R.P.; Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B Environ. 2011, 104, 330–336. [Google Scholar] [CrossRef]
- Likodimos, V.; Steriotis, T.A.; Papageorgiou, S.K.; Romanos, G.E.; Marques, R.R.N.; Rocha, R.P.; Faria, J.L.; Pereira, M.F.R.; Figueiredo, J.L.; Silva, A.M.T.; et al. Controlled surface functionalization of multiwall carbon nanotubes by HNO3 hydrothermal oxidation. Carbon 2014, 69, 311–326. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddon, R.C. Nitric acid purification of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Rocha, R.P.; Silva, A.M.T.; Dražić, G.; Pereira, M.F.R.; Figueiredo, J.L. Supported pt-particles on multi-walled carbon nanotubes with controlled surface chemistry. Mater. Lett. 2012, 66, 64–67. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Machado, B.F.; Figueiredo, J.L.; Faria, J.L. Controlling the surface chemistry of carbon xerogels using HNO3-hydrothermal oxidation. Carbon 2009, 47, 1670–1679. [Google Scholar] [CrossRef]
- Marques, R.R.N.; Machado, B.F.; Faria, J.L.; Silva, A.M.T. Controlled generation of oxygen functionalities on the surface of single-walled carbon nanotubes by HNO3 hydrothermal oxidation. Carbon 2010, 48, 1515–1523. [Google Scholar] [CrossRef]
- Rocha, R.P.; Silva, A.M.T.; Romero, S.M.M.; Pereira, M.F.R.; Figueiredo, J.L. The role of O- and S-containing surface groups on carbon nanotubes for the elimination of organic pollutants by catalytic wet air oxidation. Appl. Catal. B Environ. 2014, 147, 314–321. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Ferro-Garcia, M.A.; Joly, J.P.; Bautista-Toledo, I.; Carrasco-Marin, F.; Rivera-Utrilla, J. Activated carbon surface modifications by nitric acid, hydrogen peroxide, and ammonium peroxydisulfate treatments. Langmuir 1995, 11, 4386–4392. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Yu, D.; Nagelli, E.; Du, F.; Dai, L. Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Zhang, B.; Yu, C.; Peng, F.; Su, D. Revealing the enhanced catalytic activity of nitrogen-doped carbon nanotubes for oxidative dehydrogenation of propane. Chem. Commun. 2013, 49, 8151–8153. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Cho, K.J. Ab initio study of doped carbon nanotube sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar] [CrossRef]
- Ayala, P.; Arenal, R.; Ruemmeli, M.; Rubio, A.; Pichler, T. The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 2010, 48, 575–586. [Google Scholar] [CrossRef]
- Cuong, D.-V.; Ba, H.; Liu, Y.; Lai, T.-P.; Nhut, J.-M.; Cuong, P.-H. Nitrogen-doped carbon nanotubes on silicon carbide as a metal-free catalyst. Chin. J. Catal. 2014, 35, 906–913. [Google Scholar]
- Zhao, L.; Fan, L.-Z.; Zhou, M.-Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.-M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, E.N.; Letsoalo, P.J.; Cele, L.M.; Coville, N.J. The influence of nitrogen sources on nitrogen doped multi-walled carbon nanotubes. J. Organomet. Chem. 2010, 695, 2596–2602. [Google Scholar] [CrossRef]
- Gorgulho, H.F.; Gonçalves, F.; Pereira, M.F.R.; Figueiredo, J.L. Synthesis and characterization of nitrogen-doped carbon xerogels. Carbon 2009, 47, 2032–2039. [Google Scholar] [CrossRef]
- Pérez-Cadenas, M.; Moreno-Castilla, C.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Surface chemistry, porous texture, and morphology of N-doped carbon xerogels. Langmuir 2008, 25, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Rocha, R.P.; Gonçalves, A.G.; Pastrana-Martínez, L.M.; Bordoni, B.C.; Soares, O.S.G.P.; Órfão, J.J.M.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T.; Pereira, M.F.R. Nitrogen-doped graphene-based materials for advanced oxidation processes. Catal. Today 2015, 249, 192–198. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, L.; Wang, H.; Lv, P.; Yu, H. Sulfonated carbon nanotubes as a strong protonic acid catalyst. Carbon 2005, 43, 2405–2408. [Google Scholar] [CrossRef]
- Yu, H.; Jin, Y.; Li, Z.; Peng, F.; Wang, H. Synthesis and characterization of sulfonated single-walled carbon nanotubes and their performance as solid acid catalyst. J. Solid State Chem. 2008, 181, 432–438. [Google Scholar] [CrossRef]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Carbon as a catalyst: Esterification of acetic acid with ethanol. Catal. Today 2013, 218–219, 51–56. [Google Scholar] [CrossRef]
- Restivo, J.; Rocha, R.P.; Silva, A.M.T.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic performance of heteroatom-modified carbon nanotubes in advanced oxidation processes. Chin. J. Catal. 2014, 35, 896–905. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Hanelt, S.; Orts-Gil, G.; Friedrich, J.F.; Meyer-Plath, A. Differentiation and quantification of surface acidities on mwcnts by indirect potentiometric titration. Carbon 2011, 49, 2978–2988. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Silva, T.L.S.; Pastrana-Martinez, L.M.; Brandao, A.T.S.C.; Figueiredo, J.L.; Silva, A.M.T. Modification of the surface chemistry of single- and multi-walled carbon nanotubes by HNO3 and H2SO4 hydrothermal oxidation for application in direct contact membrane distillation. Phys. Chem. Chem. Phys. 2014, 16, 12237–12250. [Google Scholar] [CrossRef] [PubMed]
- Stüber, F.; Font, J.; Fortuny, A.; Bengoa, C.; Eftaxias, A.; Fabregat, A. Carbon materials and catalytic wet air oxidation of organic pollutants in wastewater. Top. Catal. 2005, 33, 3–50. [Google Scholar] [CrossRef]
- Santiago, M.; Stüber, F.; Fortuny, A.; Fabregat, A.; Font, J. Modified activated carbons for catalytic wet air oxidation of phenol. Carbon 2005, 43, 2134–2145. [Google Scholar] [CrossRef]
- Shende, R.V.; Mahajani, V.V. Kinetics of wet air oxidation of glyoxalic acid and oxalic acid. Ind. Eng. Chem. Res. 1994, 33, 3125–3130. [Google Scholar] [CrossRef]
- Lee, D.-K.; Kim, D.-S. Catalytic wet air oxidation of carboxylic acids at atmospheric pressure. Catal. Today 2000, 63, 249–255. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Activated carbon catalytic ozonation of oxamic and oxalic acids. Appl. Catal. B 2008, 79, 237–243. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of erythromycin over carbon materials and ceria dispersed on carbon materials. Chem. Eng. J. 2014, 250, 366–376. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: Catalytic performance and reaction pathways. J. Hazard. Mater. 2012, 239–240, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of bezafibrate promoted by carbon materials. Appl. Catal. B Environ. 2013, 140–141, 82–91. [Google Scholar] [CrossRef]
- Fan, X.; Restivo, J.; Órfão, J.J.M.; Pereira, M.F.R.; Lapkin, A.A. The role of multiwalled carbon nanotubes (MWCNTs) in the catalytic ozonation of atrazine. Chem. Eng. J. 2014, 241, 66–76. [Google Scholar] [CrossRef]
- Álvarez, P.M.; Quiñones, D.H.; Terrones, I.; Rey, A.; Beltrán, F.J. Insights into the removal of terbuthylazine from aqueous solution by several treatment methods. Water Res. 2016, 98, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, S.X.; Zhu, W.P.; Wang, J.B.; Wang, L. Catalytic wet air oxidation of phenol and aniline over multi-walled carbon nanotubes. Huanjing Kexue Environ. Sci. 2008, 29, 2522–2528. [Google Scholar]
- Yang, S.; Li, X.; Zhu, W.; Wang, J.; Descorme, C. Catalytic activity, stability and structure of multi-walled carbon nanotubes in the wet air oxidation of phenol. Carbon 2008, 46, 445–452. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, W.; Li, X.; Wang, J.; Zhou, Y. Multi-walled carbon nanotubes (MWNTs) as an efficient catalyst for catalytic wet air oxidation of phenol. Catal. Commun. 2007, 8, 2059–2063. [Google Scholar] [CrossRef]
- Soria-Sánchez, M.; Maroto-Valiente, A.; Álvarez-Rodríguez, J.; Muñoz-Andrés, V.; Rodríguez-Ramos, I.; Guerrero-Ruíz, A. Carbon nanostrutured materials as direct catalysts for phenol oxidation in aqueous phase. Appl. Catal. B Environ. 2011, 104, 101–109. [Google Scholar] [CrossRef]
- Restivo, J.; Garcia-Bordejé, E.; Órfão, J.J.M.; Pereira, M.F.R. Carbon nanofibers doped with nitrogen for the continuous catalytic ozonation of organic pollutants. Chem. Eng. J. 2016, 293, 102–111. [Google Scholar] [CrossRef]
- Qu, R.; Xu, B.; Meng, L.; Wang, L.; Wang, Z. Ozonation of indigo enhanced by carboxylated carbon nanotubes: Performance optimization, degradation products, reaction mechanism and toxicity evaluation. Water Res. 2015, 68, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Ma, J.; Cui, Y.H.; Zhang, B.P. Effect of ozonation pretreatment on the surface properties and catalytic activity of multi-walled carbon nanotube. Appl. Catal. B Environ. 2009, 92, 301–306. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Ma, J.; Cui, Y.H.; Zhao, L.; Zhang, B.P. Influence of different heat treatments on the surface properties and catalytic performance of carbon nanotube in ozonation. Appl. Catal. B Environ. 2010, 101, 74–80. [Google Scholar] [CrossRef]
- Tizaoui, C.; Mohammad-Salim, H.; Suhartono, J. Multiwalled carbon nanotubes for heterogeneous nanocatalytic ozonation. Ozone Sci. Eng. 2015, 37, 269–278. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Yang, H.; Wan, J. Catalytic wet air oxidation of phenol, nitrobenzene and aniline over the multi-walled carbon nanotubes (MWCNTs) as catalysts. Front. Environ. Sci. Eng. 2015, 9, 436–443. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Yang, H.; Sun, Y.; Liu, Y. Influence of the different oxidation treatment on the performance of multi-walled carbon nanotubes in the catalytic wet air oxidation of phenol. J. Hazard. Mater. 2012, 233–234, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of sulfamethoxazole promoted by MWCNT. Catal. Commun. 2013, 35, 82–87. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Li, F.; Lu, Y.; Liu, L.; Zhang, L.; Dai, J.; Ma, J. Relations between carbon nanotubes’ length and their composites’ mechanical and functional performance. Polymer 2013, 54, 2158–2165. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.-W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Rocha, R.P.; Restivo, J.; Sousa, J.P.S.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Nitrogen-doped carbon xerogels as catalysts for advanced oxidation processes. Catal. Today 2015, 241 Pt A, 73–79. [Google Scholar] [CrossRef]
- Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Wet air oxidation of aniline using carbon foams and fibers enriched with nitrogen. Sep. Sci. Technol. 2010, 45, 1546–1554. [Google Scholar] [CrossRef]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent progress in nitrogen-doped carbon and its composites as electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Pollak, E.; Salitra, G.; Soffer, A.; Aurbach, D. On the reaction of oxygen with nitrogen-containing and nitrogen-free carbons. Carbon 2006, 44, 3302–3307. [Google Scholar] [CrossRef]
- Szymański, G.S.; Grzybek, T.; Papp, H. Influence of nitrogen surface functionalities on the catalytic activity of activated carbon in low temperature SCR of NOx with NH3. Catal. Today 2004, 90, 51–59. [Google Scholar] [CrossRef]










| Sample | SBET | CO | CO2 | Ref. |
|---|---|---|---|---|
| (m2·g−1) | (µmol·g−1) | (µmol·g−1) | ||
| Pristine CNTs | 278 | 178 | 33 | [32] |
| CNT-HNO3 7M | --- | 1511 | 767 | |
| CNT-HNO3 HT (0.3 M) | 441 | 2015 | 680 | |
| CNT-H2O2 | 337 | 466 | 150 | [26] |
| CNT-O2 | 508 | 1339 | 91 | |
| CNT-O3 (liquid-phase) | 333 | 503 | 298 | [38] |
| CNT-HNO3-200 | 408 | 1308 | 727 | [38] |
| CNT-HNO3-400 | 408 | 1205 | 113 | |
| CNT-HNO3-600 | 389 | 826 | 43 |
| Sample | SBET | CO | CO2 | NContent | N-Functionalities | Ref. |
|---|---|---|---|---|---|---|
| (m2·g−1) | (µmol·g−1) | (µmol·g−1) | XPS/EA (wt. %) | |||
| Pristine CNTs | 291 | 200 | 23 | n.d. | --- | [50] |
| CNT-BM-M | 355 | 338 | 214 | 4.8/7.6 | N-6/N-5/N-Q | |
| CNT-BM-U | 353 | 273 | 112 | 0.8/0.9 | N-6/N-5/N-Q | |
| CNT-NUT | 386 | 703 | 76 | 0.9/0.7 | N-6/N-5 | [31] |
| Sample | SBET | CO | CO2 | SO2 | Nature of S-Groups | Ref. |
|---|---|---|---|---|---|---|
| (m2·g−1) | (µmol·g−1) | (µmol·g−1) | (µmol·g−1) | |||
| CNT-H2SO4 | --- | --- | --- | 1900 1 | –SO3H 3 | [53] |
| Pristine CNTs | 326 | 187 | 33 | --- | --- | [56] |
| CNT-H2SO4 | 294 | 381 | 195 | 579 2 | –SO3H 4 | |
| CNT-H2SO4/HNO3 | 394 | 2035 | 1394 | 203 2 | ||
| Pristine CNTs | 315 | 187 | 31 | --- | --- | [59] |
| CNT-H2SO4 HT (0.3 M) a | 437 | 1312 | 471 | 81 2 | –SO3H | |
| CNT-H2SO4 HT (0.3 M) b | --- | 625 | 164 | 53 2 |
| Process(es) | Model Compound(s) | Type of Functionalities | Reference |
|---|---|---|---|
| CWAO | Oxalic Acid/Phenol | O-/S-containing groups | [38] |
| Phenol | O-(S-) containing groups * | [71] | |
| Phenol | O-(S-) containing groups * | [72] | |
| Oxalic Acid | O-/N-containing groups | [31] | |
| Phenol | O-containing groups | [73] | |
| Phenol/Nitrobenzene/Aniline | O-containing groups | [79] | |
| Phenol | O-(S-) containing groups * | [80] | |
| CWAO/COZ | Oxalic Acid | N-containing groups | [52] |
| Oxalic Acid/Phenol | O-/N-/O-containing groups | [56] | |
| COZ | Oxalic and Oxamic Acids | O-containing groups | [26] |
| Sulfamethoxazole (antibiotic) | O-containing groups | [81] | |
| Indigo | O-containing groups | [75] | |
| Oxalic acid | O-containing groups | [76] | |
| Oxalic acid | O-containing groups | [77] | |
| Methyl orange dye | O-containing groups | [78] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, R.P.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Tuning CNT Properties for Metal-Free Environmental Catalytic Applications. C 2016, 2, 17. https://doi.org/10.3390/c2030017
Rocha RP, Soares OSGP, Figueiredo JL, Pereira MFR. Tuning CNT Properties for Metal-Free Environmental Catalytic Applications. C. 2016; 2(3):17. https://doi.org/10.3390/c2030017
Chicago/Turabian StyleRocha, Raquel P., Olívia S.G.P. Soares, José L. Figueiredo, and Manuel Fernando R. Pereira. 2016. "Tuning CNT Properties for Metal-Free Environmental Catalytic Applications" C 2, no. 3: 17. https://doi.org/10.3390/c2030017
APA StyleRocha, R. P., Soares, O. S. G. P., Figueiredo, J. L., & Pereira, M. F. R. (2016). Tuning CNT Properties for Metal-Free Environmental Catalytic Applications. C, 2(3), 17. https://doi.org/10.3390/c2030017