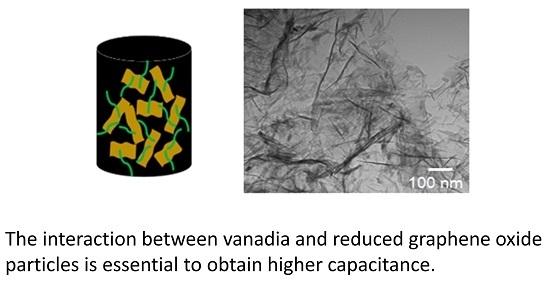
3.1. Hybrid Aerogels Produced in Method I
For hybrid aerogels produced in Method I, the concentration of VO(OC
3H
7)
3 in solution was varied from 0.64 M to 1 M. The SEM image of rGO aerogel (
Figure 1a) shows uniform morphology of randomly-arranged sheet-like structures of graphene. The SEM image of V
2O
5 aerogel presented in
Figure 1b displays 3-D networks of rod-like interconnected vanadia particles, which is further verified by the TEM image in
Figure 1c. The image in
Figure 1c suggests that the building blocks of V
2O
5 aerogel were rod-like structures of typical length approximately 300 nm and diameter several tens of nanometers. Such rod-like structures with typical length 1 µm were reported earlier [
16]. The TEM image of the hybrid aerogel rGO-V
2O
5-0.82 in
Figure 1d indicates the presence of both rod-like structure of V
2O
5 as in
Figure 1c, and the layers of thin sheets of graphene, as seen in
Figure 1a. We attribute such a hybrid aerogel structure to the specific gelation process used in Method I.
The composition of the as prepared hybrid aerogels was quantitatively determined from the TGA traces generated in air. In
Figure S2a, the TGA traces revealed the following trends: gradual weight loss at just above room temperature due to a loss of moisture; weight reduction at close to 200 °C due to the removal of bound water; and the more dramatic weight loss between 300 °C and 350 °C due to the decomposition of the organic groups on graphene [
17]. The steep decline in specimen weight at 400–430 °C was due to burning off of graphene [
11]. The weight gain after 430 °C is attributed to oxidation of V
4+ ions to V
5+ ions. The differences of the residual weights of the hybrid aerogel and that of the V
2O
5 aerogel with the same amount of vanadium isopropoxide in the formulations were used to determine the approximate graphene content in the hybrid aerogels. The graphene contents were found to be 29 wt %, 24 wt %, and 17 wt % respectively for hybrid aerogels rGO-V
2O
5-0.64, rGO-V
2O
5-0.82, and rGO-V
2O
5-1.
The crystal structure of the samples, especially the basal spacing of graphene particles, was inferred from the X-ray diffraction (XRD) patterns as shown in
Figure 2. The basal spacing of GO was found to be 0.91 nm which is much larger than that of natural graphite (0.33 nm), indicating that chemical oxidation of graphite to GO was complete in these materials and that the interlayer distance was expanded [
18]. In the case of rGO aerogel, the XRD pattern shows a broad peak at around 24°. In addition, the peak at 9.8° corresponding to (001) plane of GO is absent in
Figure 2a. This indicates that the method of reduction of GO to graphene using L-ascorbic acid was successful.
The XRD pattern of the rGO-V
2O
5-0.82 hybrid aerogel revealed only two reflection peaks at around 7.4° and 27.9°, and the low intensity of the peaks suggests a small degree of crystalline order [
19]. In view of previous work [
20,
21], the (001) peak at 7.4° corresponds to the layer stacking of V
2O
5 (d = 1.19 nm) [
22,
23]. The absence of a graphene peak in the XRD patterns of hybrid aerogels indicates that restacking of graphene sheets did not occur in hybrid aerogels and that graphene was present mostly as single sheets [
24]. The FTIR spectra shown in
Figure S3 suggests the chemical structures of GO, rGO, and the hybrid aerogels.
Raman spectroscopy was used to determine the quality of graphene by analyzing the intensity ratio of the D- and G-bands. The D-band results from the disorder-induced mode reflecting structural defects and the G-band is associated with the E
2g mode from sp
2-hybridized carbon domains [
25]. The intensity ratio I
D/I
G reflects the disorder degree of carbon materials [
26]. The Raman spectra of GO and rGO aerogel are shown in
Figure 2c. There are two obvious peaks at around 1357 cm
−1 and 1608 cm
−1 in the Raman spectrum of GO corresponding to the D-band and G-band, respectively. The Raman spectrum of rGO aerogel also shows the D- (at 1343 cm
−1) and G-band (at 1585 cm
−1). The I
D/I
G ratio of rGO aerogel is 1.24 which is greater than that of GO (1.15) indicating a reduction of the average size of the sp
2 domains [
27] when the oxygen groups were removed during the reduction process of GO. In addition, the width of the half-maximum of the G-band was reduced, which suggests a high graphitization degree of rGO aerogel [
28].
The presence of platelet-type graphene particles and rod-like vanadia particles in the hybrid aerogels presents several interesting scenarios in terms of solid surface area and pore size distribution. Barrett–Joyner–Halenda (BJH) pore size distributions of rGO aerogel and rGO-V
2O
5-0.82 hybrid aerogel are presented in
Figure S4a,b. These were obtained from nitrogen adsorption-desorption isotherms shown in
Figure S5. As is evident, the isotherms in
Figure S5 are type IV, indicating mesoporous structures. Both samples show mesopores with distinct pore size distribution, as seen in
Figure S4. In the case of rGO aerogel, the predominant pores are 4 nm and 60 nm, while those for rGO-V
2O
5-0.82 hybrid aerogel are 4 nm and 40 nm, the latter due to the presence of V
2O
5. The BET specific surface area of rGO-V
2O
5 hybrid aerogels are generally lower than that of rGO aerogel (641 m
2/g), e.g., 396, 348, 299 m
2/g, respectively, for hybrid aerogel specimens rGO-V
2O
5-0.64, rGO-V
2O
5-0.82, and rGO-V
2O
5-1. Note that the specific surface area of V
2O
5 aerogel (254 m
2/g) is much smaller than that of rGO aerogel.
The specific capacitance of the electrodes fabricated using V
2O
5 aerogel, rGO aerogel, and V
2O
5-rGO hybrid aerogels were determined from galvanostatic charge/discharge test data. The values of specific capacitance are shown in
Figure 3a. We find that the specific capacitance of the rGO-V
2O
5-0.82 hybrid aerogel calculated from charge/discharge curves are much higher (203 F/g), compared to the rGO aerogel (105 F/g) and the V
2O
5 aerogel (132 F/g). In view of this, the electrochemical performance the rGO-V
2O
5-0.82 hybrid aerogel was further investigated. In
Figure 3b, the CV curve of the rGO aerogel exhibits an almost rectangular shape suggesting the EDLC characteristic [
3]. In the case of the rGO-V
2O
5-0.82 hybrid aerogel, a pair of redox peaks are observed with a larger area than the V
2O
5 aerogel and the graphene aerogel under the same scanning rate. These indicate that the specific capacitance of the rGO-V
2O
5-0.82 hybrid aerogel is higher than both the rGO and V
2O
5 aerogels along with a Faradaic redox reaction occurring during the charge/discharge process (
Figure 3c) [
3]. Furthermore, for the hybrid aerogel system, the redox peak is more well-defined, which implies that the presence of graphene improves the utilization of V
2O
5 and, therefore, contributes pseudo-capacitance to the overall capacitance. The CV curves of the rGO-V
2O
5-0.82 hybrid aerogel at varied scan rates (
Figure 3d) show a regular and symmetric shape to zero-current line indicating an excellent electrochemical reversibility due to the synergetic effect of EDLC and pseudo-capacitor. The Nyquist plot shown in
Figure 3e reveals that rGO aerogel shows an almost ideal EDLC feature with a nearly vertical straight line along the imaginary axis in the low frequency region. Additionally, for the rGO-V
2O
5-0.82 hybrid aerogel, a stronger vertical line than the V
2O
5 aerogel are observed, which is closer to the line for the rGO aerogel. Moreover, the charge transfer resistance (R
CT) measured by the diameter of the semicircle in the high-frequency region also reflects the capacitive property [
29]. The calculated values of R
CT for the rGO aerogel, the V
2O
5 aerogel, and the rGO-V
2O
5-0.82 hybrid aerogel are 0.4 Ω, 3.7 Ω, and 1.7 Ω, respectively. These results indicate that the combination of V
2O
5 and graphene improves the conductivity of the electrode materials and minimizes the ion transportation path.
The cycle stability was tested at 10 A/g of charge/discharge for 1000 cycles (
Figure 3f). It can be seen that the degradation of specific capacitance of the rGO aerogel starts from 100 cycles with a consistent, but small, reduction until 1000 cycles exhibiting a characteristic of EDLC. Moreover, the V
2O
5 aerogel shows a quite poor cycle stability due to the structural damage caused by the mechanical stress induced by the ion intercalation-de-intercalation phenomena during the charge-discharge process [
30] and dissolution of vanadium oxide [
31]. However, the cycle stability of the rGO-V
2O
5-0.82 hybrid aerogel obviously improved, e.g., 73% of initial capacitance. This was achieved due to the presence of graphene nanosheets that were able to withstand the structural changes during the charge-discharge process.
3.2. Hybrid Aerogels from One-Pot Synthesis via Method II
The SEM and TEM images (
Figure 1e,f) of the heat-treated V
2O
5 aerogel reveal a randomly-arranged rod-like morphology with lengths less than 100 nm and diameters of several nanometers. These dimensions are much lower than those of the V
2O
5 aerogel and can be attributed to crystallization at the time of thermal treatment. The V
2O
5-rGO-6 hybrid aerogel displays a morphology of crumpled graphene sheets where the growth of V
2O
5 nanofibers is apparent (
Figure 1g,h). We note that GO particles were well-dispersed in water and the V
2O
5 nanofibers possibly grew on the surfaces of graphene sheets. The morphology of the V
2O
5-rGO-6 hybrid aerogel further confirms the interaction of VO oligomers with GO sheets in the hydrolysis step.
Figure S2b shows the TGA traces of the samples conducted in air with a heating rate of 10 °C/min. The weight loss of the V
2O
5 aerogel before 150 °C is due to the evaporation of absorbed moisture. In the case of the V
2O
5-rGO hybrid aerogels, there is gradual weight loss from around room temperature to about 400 °C, suggesting that such loss was due to the evaporation of moisture and decomposition of the oxygen-containing groups of GO. In addition, for all hybrid aerogels, a sharp weight reduction occurred in the range of 400 °C to 480 °C, due to the burning of graphene [
32]. The graphene weight percent in hybrid aerogels V
2O
5-rGO-2, V
2O
5-rGO-4, and V
2O
5-rGO-6 was estimated to be 12%, 17%, and 23%, respectively.
The X-ray diffraction patterns of the heat-treated V
2O
5 aerogel and V
2O
5-rGO hybrid aerogels (
Figure 4a,b) were examined to determine if the heat treatment step used for thermal reduction of GO also caused substantial changes in the crystal structures of the hybrid materials. In
Figure 4b, the XRD patterns of a series of V
2O
5-rGO hybrid aerogels exhibit (200), (001), (110), (301), and (310) reflection peaks corresponding to orthorhombic crystalline V
2O
5 (JCPDS No. 41-1426) at the same position as in the case of V
2O
5 aerogel (
Figure 4a). These indicate that the V
2O
5-rGO hybrid aerogels were partly crystalline due to the thermal treatment. Additionally, in comparison to V
2O
5 aerogel, no additional peaks are detected in the hybrid aerogels, which indicates high purity of the V
2O
5 parts in the aerogel. The intensity of the XRD reflection peaks shows reduction with an increase of the amount of graphene in the V
2O
5-rGO hybrid aerogels while the peak positions remain the same. This implies that the V
2O
5 layered structure was maintained despite the presence of graphene, although, graphene influenced its crystal formation. The XRD patterns only show V
2O
5 reflection peaks in the V
2O
5-rGO hybrid aerogels, which indicates that V
2O
5 nanofibers prevented restacking of graphene nanosheets [
22].
Figure 4c shows FTIR spectra to verify if the thermal reduction method successfully reduced GO to graphene. The characteristic peaks in FTIR spectra at 569, 834, and 1018 cm
−1 of the V
2O
5 aerogel correspond to triply-coordinated oxygen (chain oxygen) bonds in vanadium oxide, doubly-coordinated oxygen (bridge oxygen) bonds, and stretching vibration of terminal oxygen bonds (V=O), respectively [
24]. For V
2O
5-rGO-6 hybrid aerogel, the vanadium oxide characteristic peaks shifted, indicating an interaction of V
2O
5 nanofibers and graphene nanosheets [
33]. The bond at 1599 cm
−1 is observed clearly due to the skeletal vibration of graphene [
34].
In Raman spectra shown in
Figure 4d,e, the peaks at 144, 284, 406, 522, 699, and 995 cm
−1 can be observed in both the V
2O
5 aerogel and the V
2O
5-rGO-6 hybrid aerogel, which correspond to the following signatures: skeleton bending vibration of the V-O-V bonds (144 cm
−1), bending vibrations of the V=O bonds (284 cm
−1), bending vibrations of bridge oxygen bonds (406 cm
−1), stretching vibrations of triply-coordinated oxygen bonds (522 cm
−1), stretching vibrations of doubly-coordinated oxygen (699 cm
−1), and in-phase stretching vibrational of V=O bonds (995 cm
−1) [
13]. The two peaks at 1359 and 1601 cm
−1 in the V
2O
5-rGO-6 hybrid aerogel belong to the D- and G-bands of graphene, which suggests a complete reduction of GO and the presence of graphene.
The BJH pore distribution (
Figure S4c) of V
2O
5-rGO hybrid aerogels exhibits slightly different distribution from the V
2O
5 aerogel, especially the V
2O
5-rGO-6 hybrid aerogel shows a peak at 40 nm. This pore size distribution of the two types of samples might have some effect on the electrochemical property because the mesopores provide more accessibility for ion transportation [
35]. The BET-specific surface areas of the samples are listed in
Table 1 and
Table 2.
The BET-specific surface area of the V
2O
5-rGO-6 hybrid aerogel (392 cm
2/g) is much larger than the V
2O
5 aerogel (213 cm
2/g), which resulted from the presence of high surface area graphene nanosheets. This has the potential to provide much higher capacity for energy storage. Note in this case that the surface area of V
2O
5 aerogel listed in
Table 2 is lower than that reported in
Table 1. After annealing, the pore structure of the V
2O
5 aerogel changed due to the oxidation of VO
x and partial crystallization of V
2O
5. Thus, the BET-specific surface area of the heat-treated V
2O
5 aerogel reduced to 213 m
2·g
−1. For a supercapacitor, especially for hybrid electrodes, the surface area serves as a significant factor for charge storage that provides space for Faradaic redox reactions to produce pseudocapacitance. In this way, the large specific surface area of the V
2O
5-rGO hybrid aerogel favors capacitance.
The specific capacitance was obtained from the data of charge-discharge curves and calculated from Equation (1). The galvanostatic charge/discharge curves of the different V
2O
5-rGO hybrid aerogels are shown in
Figure 5a. It can be seen that the specific capacitance of the V
2O
5-rGO hybrid aerogel is generally higher than the V
2O
5 aerogel (98 F/g). In addition, an increase in specific capacitance is observed for materials with higher content of graphene in the V
2O
5-rGO hybrid aerogels. The specific capacitance of a series of V
2O
5-rGO hybrid aerogels, respectively, for the V
2O
5-rGO-2, V
2O
5-rGO-4, and V
2O
5-rGO-6 hybrid aerogels are 163, 202, and 272 F/g, which are summarized in
Figure 5b. This tendency is led by a synergetic effect of V
2O
5 and graphene, in which graphene enhances the conductivity and increases the specific surface area of the V
2O
5-rGO hybrid aerogels in order to promote better utilization of V
2O
5.
Since the specific capacitance of the V
2O
5-rGO-6 hybrid aerogel is the highest among the three materials studied, further investigation of its electrochemical performance was conducted. The cyclic voltammetry curves of both samples are shown in
Figure 5c. The shape of CV curves of both materials is symmetrical, suggesting an electrochemical reversibility. These curves deviated from an ideal rectangle indicating the Faradic pseudocapacitance. A pair of redox peaks of potentials at around 0.32 V and 0.30 V resulted from the transformation between V
5+ and V
4+. However, the CV curve of the V
2O
5-rGO-6 hybrid aerogel shows undefined redox peaks, which are possibly caused by the EDLC contribution by graphene. This result further confirms the synergetic effect of the double-layer capacitance and pseudocapacitance [
36]. The area of the CV curve of the V
2O
5-rGO-6 hybrid aerogel is much larger than that of the V
2O
5 aerogel, and this also confirms a higher specific capacitance of the V
2O
5-rGO-6 hybrid aerogel. The CV curves of the V
2O
5-rGO-6 hybrid aerogel at different scan rates are shown in
Figure 5d in order to further study the capacitive performance. As the scan rate increases, the quasi-rectangular shape of the CV curves is affected, although the shape of the curves remains mirror-symmetric with zero-current line even at the high scan rate of 100 mV/s. This result implies a high rate performance and good capacitive reversibility [
37]. Note that the specific capacitance of the V
2O
5-rGO-6 hybrid aerogel is much higher than that of graphene-templated rGO-V
2O
5-0.82 hybrid aerogel (203 F·g
−1) produced in Method I with a similar amount of graphene, even if V
2O
5 is partly crystalline due to the thermal reduction for the V
2O
5-rGO-6 hybrid aerogel.
It can be concluded from the data presented up to this point that the preparation method strongly influenced the structure-properties relationships and the final electrochemical performance. In Method I, the capillary force contributed to selective localization of V2O5 on the graphene nanosheets. Additionally, the unavoidable diffusion gradient quite possibly led to a heterogeneous dispersion of V2O5 within the rGO aerogel produced by Method I. In Method II, the recombination of V2O5 and graphene in hybrid aerogels was easily accomplished and enhanced by the coordination bonds between V2O5 and graphene nanosheets that are much stronger than the capillary force in Method I. Meanwhile, the uniform mixing of V2O5 and graphene also produces a homogeneous hybrid structure in Method II. In addition, the BET-specific surface area of the V2O5-rGO-6 hybrid aerogel is greater than the rGO-V2O5-0.82 hybrid aerogel, suggesting that there is more capacity for energy storage in hybrid materials produced by Method II. These factors answer why the hybrid aerogels from Method I exhibit inferior electrochemical performance than hybrid aerogels produced by Method II.
The Nyquist plots of EIS of V
2O
5 aerogel are shown in
Figure 5e. The corresponding equivalent circuit is presented in
Figure S6. In comparison, the V
2O
5-rGO-6 hybrid aerogel has more vertical lines parallel to the imaginary axis than the V
2O
5 aerogel, which indicates that the electrical conductivity of the hybrid aerogel was improved or the accessibility of ions in the electrolyte was enhanced due to the combination of the structures from graphene and V
2O
5 and, therefore, exhibited a behavior more closely to that of an ideal supercapacitor. The cycle stabilities of the V
2O
5 aerogel and the V
2O
5-rGO-6 hybrid aerogel were characterized by a charge/discharge test over 1000 cycles at a constant current density of 10 A/g shown in
Figure 5f. This indicates clearly that the capacitance retention of the V
2O
5-rGO-6 hybrid aerogel was 80% after 1000 operation cycles, while the capacitance retention of the V
2O
5 aerogel was only 35% of the initial specific capacitance. The low capacitance retention of the V
2O
5 aerogel can be attributed to the structural damage caused by the insertion and desertion of electrolyte ions during the charge/discharge process [
38] and two clear drops after 350th and 700th cycles could be possibly caused by the large structural changes of V
2O
5. According to published literature, pure V
2O
5 shows very poor cycle stability up to only 100 cycles in aqueous electrolyte [
19]. Obviously, the addition of graphene improved the cycle stability for the materials developed in this work due to its electroactive property.