Effects of Ion Bombardment Energy Flux on Chemical Compositions and Structures of Hydrogenated Amorphous Carbon Films Grown by a Radical-Injection Plasma-Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Plasma Characteristics
3.2. Effects of Ion Bombardment Energy Flux on the Deposition Rate
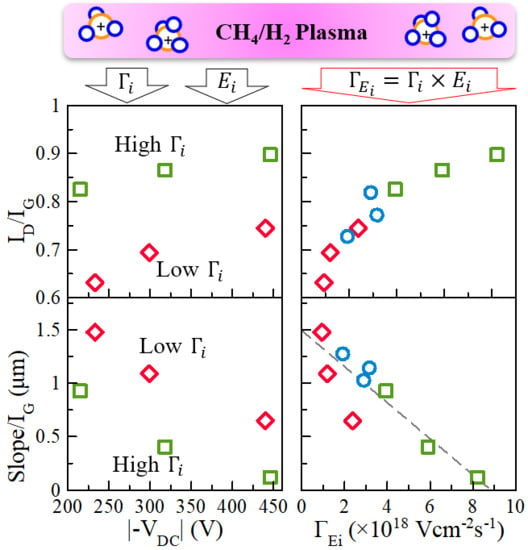
3.3. Effects of Ion Bombardment Energy Flux on the Incorpolation of sp2-C Clusters, the Slope/IG, and the Density of Dangling Bonds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ray, S.C.; Mukherjee, D.; Sarma, S.; Bhattacharya, G.; Mathur, A.; Roy, S.S.; McLaughlin, J.A. Functional diamond like carbon (DLC) coatings on polymer for improved gas barrier performance. Diam. Relat. Mater. 2017, 80, 59–63. [Google Scholar] [CrossRef]
- Koós, M.; Füle, M.; Veres, M.; Tóth, S.; Pócsik, I. Multi-band structure of amorphous carbon luminescence. Diam. Relat. Mater. 2002, 11, 1115–1118. [Google Scholar] [CrossRef]
- Gerstner, E.G.; Mckenzie, D.R. Fabrication and characterization of novel electronic devices using tetrahedral amorphous carbon. Diam. Relat. Mater. 2006, 7, 1172–1177. [Google Scholar] [CrossRef]
- Adhikari, S.; Adhikary, S.; Omer, A.M.M.; Rusop, M.; Uchida, H.; Soga, T.; Umeno, M. Optical and structural properties of amorphous carbon thin films deposited by microwave surface-wave plasma CVD. Diam. Relat. Mater. 2006, 15, 188–192. [Google Scholar] [CrossRef]
- Andujar, J.L.; Polo, M.C.; Esteve, J.; Robertson, J.; Milne, W.I.; Martínez, E. Study of the mechanical properties of tetrahedral amorphous carbon films by nanoindentation and nanowear measurements. Diam. Relat. Mater. 2001, 10, 145–152. [Google Scholar] [CrossRef]
- Tiainen, V. Amorphous carbon as a bio-mechanical coating—Mechanical properties and biological applications. Diam. Relat. Mater. 2001, 10, 153–160. [Google Scholar] [CrossRef]
- Kanda, K.; Fukuda, K.; Kidena, K.; Imai, R.; Niibe, M.; Fujimoto, S.; Yokota, K.; Tagawa, M. Hyperthermal atomic oxygen beam irradiation effect on the Ti-containing DLC film. Diam. Relat. Mater. 2014, 41, 49–52. [Google Scholar] [CrossRef]
- Wazumi, K.; Koga, Y.; Tanaka, A. Tribological properties of a-C:H films on Si substrate prepared by plasma CVD in pulse-biased process. Diam. Relat. Mater. 2003, 12, 1018–1023. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Marchon, B.; Gui, J.; Grannen, K.; Rauch, G.C.; Ager, J.W.; Silva, S.R.P.; Robertson, J. Photoluminescence and Raman spectroscopy in hydrogenated carbon films. IEEE Trans. Magn. 1997, 33, 3148–3150. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J. Plasma Deposition of Diamond-Like Carbon. Jpn. J. Appl. Phys. 2011, 50, 01AF01. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Kasi, S.R.; Rabalais, J.W.; Eckstein, W. Subplantation model for film growth from hyperthermal species. Phys. Rev. B 1990, 41, 10468–10480. [Google Scholar] [CrossRef]
- Robertson, J. Deposition mechanisms for promoting sp3 bonding in diamond-like carbon. Diam. Relat. Mater. 1993, 2, 984–989. [Google Scholar] [CrossRef]
- Möller, W. Modelling and computer simulation of ion-beam- and plasma-assisted film growth. Thin Solid Films 1993, 228, 319–325. [Google Scholar] [CrossRef]
- Robertson, J. The deposition mechanism of diamond-like a-C and a-C: H. Diam. Relat. Mater. 1994, 3, 361–368. [Google Scholar] [CrossRef]
- Davis, C.A. A simple model for the formation of compressive stress in thin films by ion bombardment. Thin Solid Films 1993, 226, 30–34. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Nakadate, H.; Ikeyama, M.; Nakao, S.; Miyagawa, S. Dynamic MC simulation for a-C:H deposition in methane plasma based on subplantation model. Diam. Relat. Mater. 2003, 12, 927–930. [Google Scholar] [CrossRef]
- Godet, C.; Conway, N.M.J.; Bourée, J.E.; Bouamra, K.; Grosman, A.; Ortega, C. Structural and electronic properties of electron cyclotron resonance plasma deposited hydrogenated amorphous carbon and carbon nitride films. J. Appl. Phys. 2002, 91, 4154. [Google Scholar] [CrossRef]
- Oda, A.; Kousaka, H. Numerical Analysis of Substrate-Incident Carbon Flux in Low-Pressure Radio-Frequency CH 4 Plasmas for Deposition of Diamond-Like Carbon Films. Electron. Commun. Jpn. 2015, 98, 31–39. [Google Scholar] [CrossRef]
- Sugiura, H.; Jia, L.; Kondo, H.; Ishikawa, K.; Tsutsumi, T.; Hayashi, T.; Takeda, K.; Sekine, M.; Hori, M. Effects of gas residence time of CH4/H2 on sp2 fraction of amorphous carbon films and dissociated methyl density during radical-injection plasma-enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2018, 57, 06JE03. [Google Scholar] [CrossRef]
- Jia, L.; Sugiura, H.; Kondo, H.; Takeda, K.; Ishikawa, K.; Oda, O.; Sekine, M.; Hiramatsu, M.; Hori, M. Effects of Radical Species on Structural and Electronic Properties of Amorphous Carbon Films Deposited by Radical-Injection Plasma-Enhanced Chemical Vapor Deposition. Plasma Process. Polym. 2016, 13, 730–736. [Google Scholar] [CrossRef]
- Kondo, S.; Hori, M.; Yamakawa, K.; Den, S.; Kano, H.; Hiramatsu, M. Highly reliable growth process of carbon nanowalls using radical injection plasma-enhanced chemical vapor deposition. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2008, 26, 1294. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Kondo, H.; Hori, M. New Progress on Graphene Research; IntechOpen: London, UK, 2013. [Google Scholar]
- Mankelevich, Y.A.; May, P.W. New insights into the mechanism of CVD diamond growth: Single crystal diamond in MW PECVD reactors. Diam. Relat. Mater. 2008, 17, 1021–1028. [Google Scholar] [CrossRef]
- Toyota, H.; Nomura, S.; Mukasa, S.; Yamashita, H.; Shimo, T.; Okuda, S. A consideration of ternary C–H–O diagram for diamond deposition using microwave in-liquid and gas phase plasma. Diam. Relat. Mater. 2011, 20, 1255–1258. [Google Scholar] [CrossRef]
- Ueyama, T.; Fukunaga, Y.; Tsutsumi, T.; Takeda, K.; Kondo, H.; Ishikawa, K.; Sekine, M.; Iwata, M.; Ohya, Y.; Sugai, H.; et al. Electron behaviors in afterglow of synchronized dc-imposed pulsed fluorocarbon-based plasmas. Jpn. J. Appl. Phys. 2017, 56, 06HC03. [Google Scholar] [CrossRef] [Green Version]
- Ohya, Y.; Ishikawa, K.; Komuro, T.; Yamaguchi, T.; Takeda, K.; Kondo, H.; Sekine, M.; Hori, M. Spatial profiles of interelectrode electron density in direct current superposed dual-frequency capacitively coupled plasmas. J. Phys. D. Appl. Phys. 2017, 50, 155201. [Google Scholar] [CrossRef]
- Ohya, Y.; Iwata, M.; Ishikawa, K.; Sekine, M.; Hori, M.; Sugai, H. Rapid electron density decay observed by surface-wave probe in afterglow of pulsed fluorocarbon-based plasma. Jpn. J. Appl. Phys. 2016, 55, 080309. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Komuro, T.; Koshimizu, C.; Takashima, S.; Takeda, K.; Kondo, H.; Ishikawa, K.; Sekine, M.; Hori, M. Direct current superposed dual-frequency capacitively coupled plasmas in selective etching of SiOCH over SiC. J. Phys. D. Appl. Phys. 2012, 45, 025203. [Google Scholar] [CrossRef]
- Kokura, H.; Nakamura, K.; Ghanashev, I.P.; Sugai, H. Plasma Absorption Probe for Measuring Electron Density in an Environment Soiled with Processing Plasmas. Jpn. J. Appl. Phys. 1999, 38, 5262–5266. [Google Scholar] [CrossRef]
- Park, C. Hydrogen line ratios as electron temperature indicators in nonequilibrium plasmas. J. Quant. Spectrosc. Radiat. Transf. 1972, 12, 323–370. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Rose, F.; Wang, N.; Smith, R.; Xiao, Q.-F.; Inaba, H.; Matsumura, T.; Saito, Y.; Matsumoto, H.; Dai, Q.; Marchon, B.; et al. Complete characterization by Raman spectroscopy of the structural properties of thin hydrogenated diamond-like carbon films exposed to rapid thermal annealing. J. Appl. Phys. 2014, 116, 123516. [Google Scholar] [CrossRef] [Green Version]
- Buijnsters, J.G.; Gago, R.; Jiménez, I.; Camero, M.; Agulló-Rueda, F.; Gómez-Aleixandre, C. Hydrogen quantification in hydrogenated amorphous carbon films by infrared, Raman, and X-ray absorption near edge spectroscopies. J. Appl. Phys. 2009, 105, 093510. [Google Scholar] [CrossRef]
- Colgan, M.J.; Meyyappan, M.; Murnick, D.E. Very high-frequency capacitively coupled argon discharges. Plasma Sources Sci. Technol. 1994, 3, 181–189. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Zhou, X.; Suzuki, T.; Nakajima, H.; Komatsu, K.; Kanda, K.; Ito, H.; Saitoh, H. Structural analysis of amorphous carbon films by spectroscopic ellipsometry, RBS/ERDA, and NEXAFS. Appl. Phys. Lett. 2017, 110, 201902. [Google Scholar] [CrossRef] [Green Version]
- Adamopoulos, G.; Robertson, J.; Morrison, N.A.; Godet, C. Hydrogen content estimation of hydrogenated amorphous carbon by visible Raman spectroscopy. J. Appl. Phys. 2004, 96, 6348. [Google Scholar] [CrossRef]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiura, H.; Kondo, H.; Tsutsumi, T.; Ishikawa, K.; Hori, M. Effects of Ion Bombardment Energy Flux on Chemical Compositions and Structures of Hydrogenated Amorphous Carbon Films Grown by a Radical-Injection Plasma-Enhanced Chemical Vapor Deposition. C 2019, 5, 8. https://doi.org/10.3390/c5010008
Sugiura H, Kondo H, Tsutsumi T, Ishikawa K, Hori M. Effects of Ion Bombardment Energy Flux on Chemical Compositions and Structures of Hydrogenated Amorphous Carbon Films Grown by a Radical-Injection Plasma-Enhanced Chemical Vapor Deposition. C. 2019; 5(1):8. https://doi.org/10.3390/c5010008
Chicago/Turabian StyleSugiura, Hirotsugu, Hiroki Kondo, Takayoshi Tsutsumi, Kenji Ishikawa, and Masaru Hori. 2019. "Effects of Ion Bombardment Energy Flux on Chemical Compositions and Structures of Hydrogenated Amorphous Carbon Films Grown by a Radical-Injection Plasma-Enhanced Chemical Vapor Deposition" C 5, no. 1: 8. https://doi.org/10.3390/c5010008
APA StyleSugiura, H., Kondo, H., Tsutsumi, T., Ishikawa, K., & Hori, M. (2019). Effects of Ion Bombardment Energy Flux on Chemical Compositions and Structures of Hydrogenated Amorphous Carbon Films Grown by a Radical-Injection Plasma-Enhanced Chemical Vapor Deposition. C, 5(1), 8. https://doi.org/10.3390/c5010008