Bioimaging Applications of Carbon Nanodots: A Review
Abstract
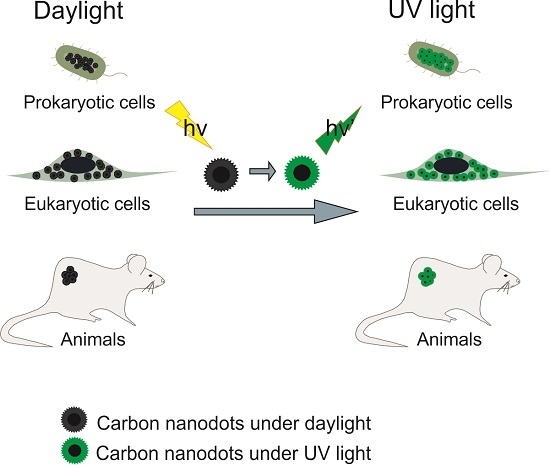
:1. Introduction
2. Synthesis of CNDs
3. Imaging Applications for Bacteria and Fungi
4. Imaging Applications for Eukaryotic Cells
5. Target-Specific Imaging Applications for Eukaryotic Cells
6. In Vivo Imaging Applications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Baker, G. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, K.; Tang, Z.; Xu, Y. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Du, F.; Zeng, F.; Ming, Y.; Wu, S. Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim. Acta 2013, 180, 453–460. [Google Scholar] [CrossRef]
- Yang, K.; Liu, M.; Wang, Y.; Wang, S.; Miao, H.; Yang, L.; Yang, X. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Huang, H.; Li, C.; Zhu, S.; Wang, H.; Chen, C.; Wang, Z.; Bai, T.; Shi, Z.; Feng, S. Histidine-derived nontoxic nitrogen-doped carbon dots for sensing and bioimaging applications. Langmuir 2014, 30, 13542–13548. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Roy, A.; Kim, S.; Paoprasert, P.; Park, S.; In, I. Preparation of biocompatible and antibacterial carbon quantum dots derived from resorcinol and formaldehyde spheres. RSC Adv. 2015, 5, 31677–31682. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Che, Y.; Liao, X.; Jiang, Y. A type of novel fluorescent magnetic carbon quantum dots for cells imaging and detection. J. Biomed. Mater. Res. A 2015, 103, 3956–3964. [Google Scholar] [CrossRef]
- Matea, C.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Kuan, C.; Lin, C.; Wang, L.; Chang, C.; Wang, T. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016, 6, 21170. [Google Scholar] [CrossRef]
- Hassan, M.; Gomes, V.; Dehghani, A.; Ardekani, S. Engineering carbon quantum dots for photomediated theranostics. Nano Res. 2018, 11, 1–41. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef]
- Chatzimarkou, A.; Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Stalikas, C. Selective FRET-based sensing of 4-nitrophenol and cell imaging capitalizing on the fluorescent properties of carbon nanodots from apple seeds. Sens. Actuators B 2018, 258, 1152–1160. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Leonardos, I.; Troganis, A.; Stalikas, C. Human fingernails as an intriguing precursor for the synthesis of nitrogen and sulfur-doped carbon dots with strong fluorescent properties: Analytical and bioimaging applications. Sens. Actuators B 2018, 267, 494–501. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Troganis, A.; Stalikas, C. Carbonization of human fingernails: Toward the sustainable production of multifunctional nitrogen and sulfur codoped carbon nanodots with highly luminescent probing and cell proliferative/migration properties. ACS Appl. Mater. Interfaces 2018, 10, 16024–16032. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Shi, L.; Wen, G.; Li, Y.; Dong, C.; Yang, J.; Shuang, S. Green synthesis of carbon nanodots from cotton for multicolor imaging, patterning, and sensing. Sens. Actuators B 2015, 221, 769–776. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, S.; Yang, Y.; Wu, Y.; Wang, X.; Wu, J.; Zhang, L.; Chen, J.; Wang, Y. A facile synthesis of highly nitrogen-doped carbon dots for imaging and detection in biological samples. J. Anal. Methods Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, M.; Park, S.; Zhang, Y.; Akanda, M.; Park, B.-Y.; Kim, H. Green synthesis of fluorescent carbon dots from carrot juice for in vitro cellular imaging. Carbon Lett. 2017, 21, 61–67. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Z.; Gong, A.; Zhang, Y.; Li, Q. Synthesis of highly photoluminescent carbon dots via citric acid and Tris for iron(III) ions sensors and bioimaging. Talanta 2015, 143, 107–113. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Chen, M.; Chen, X.; Wang, J. The production of pH-sensitive photoluminescent carbon nanoparticles by the carbonization of polyethylenimine and their use for bioimaging. Carbon 2013, 55, 343–349. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Li, S.; Weng, W.; Guo, H.; Guo, T.; Zhang, X.; Chen, Y.; Huang, T.; Hong, X.; et al. Large scale synthesis of photoluminescent carbon nanodots and their application for bioimaging. Nanoscale 2013, 5, 1967–1971. [Google Scholar] [CrossRef]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon Dots Prepared by Hydrothermal Treatment of Dopamine as an Effective Fluorescent Sensing Platform for the Label-Free Detection of Iron(III) Ions and Dopamine. Chem.–Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef]
- Hsu, P.-; Shih, Z.-; Lee, C.-H.; Chang, H. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Tian, F.; Li, W.; Li, F.; Liu, W. One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 2011, 21, 13163–13167. [Google Scholar] [CrossRef]
- Xinyue, Z.; Mingyue, J.; Na, N.; Zhijun, C.; Shujun, L.; Shouxin, L.; Jian, L. Natural-product-derived carbon dots: From natural products to functional materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef]
- Zheng, X.; Ananthanarayanan, A.; Luo, K.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Das, P.; Bose, M.; Ganguly, S.; Mondal, S.; Das, A.; Banerjee, S.; Das, N. Green approach to photoluminescent carbon dots for imaging of gram-negative bacteriaEscherichia coli. Nanotechnology 2017, 28, 195501. [Google Scholar] [CrossRef]
- Pal, T.; Mohiyuddin, S.; Packirisamy, G. Facile and green synthesis of multicolor fluorescence carbon dots from curcumin: In vitro and in vivo bioimaging and other applications. ACS Omega 2018, 3, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.; Chen, Y. Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef]
- Pathak, A.; Pv, S.; Stanley, J.; Satheesh Babu, T. Multicolor emitting N/S-doped carbon dots as a fluorescent probe for imaging pathogenic bacteria and human buccal epithelial cells. Microchim. Acta 2019, 186, 157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Milcovich, G.; Chen, T.; Durack, E.; Mallen, S.; Yongming, R.; Weng, X.; Hudson, S. Co-reductive fabrication of carbon nanodots with high quantum yield for bioimaging of bacteria. Beilstein J. Nanotechnol. 2018, 9, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Ma, Y.; Gao, G.; Chen, X.; Jia, H.-R.; Li, Y.; Chen, Z.; Wu, F. Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef]
- Nandi, S.; Ritenberg, M.; Jelinek, R. Bacterial detection with amphiphilic carbon dots. Analyst 2015, 140, 4232–4237. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Song, Y.; Huang, H.; Liu, Y.; Fu, Y.; Huang, J.; Li, H.; Qu, H.; Kang, Z. Fluorescent carbon dots with tunable negative charges for bio-imaging in bacterial viability assessment. Carbon 2017, 120, 95–102. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Lu, F.; Wang, H.; Zhang, M.; Yang, J.; Huang, J.; Huang, H.; Liu, Y.; Kang, Z. Fluorescent carbon dots with highly negative charges as a sensitive probe for real-time monitoring of bacterial viability. J. Mater. Chem. B 2017, 5, 6008–6015. [Google Scholar] [CrossRef]
- Hua, X.; Bao, Y.; Wang, H.; Chen, Z.; Wu, F. Bacteria-derived fluorescent carbon dots for microbial live/dead differentiation. Nanoscale 2017, 9, 2150–2161. [Google Scholar] [CrossRef]
- Bhamore, J.; Jha, S.; Park, T.; Kailasa, S. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. B 2019, 191, 150–155. [Google Scholar] [CrossRef]
- Kasibabu, B.; D’Souza, S.; Jha, S.; Singhal, R.; Basu, H.; Kailasa, S. One-step synthesis of fluorescent carbon dots for imaging bacterial and fungal cells. Anal. Methods 2015, 7, 2373–2378. [Google Scholar] [CrossRef]
- Kasibabu, B.; D’souza, S.; Jha, S.; Kailasa, S. Imaging of bacterial and fungal cells using fluorescent carbon dots prepared from Carica papaya juice. J. Fluoresc. 2015, 25, 803–810. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.; Zboril, R.; Rogach, A. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Hua, Y.; Zhang, M.; Zhou, Z.; Yuan, J.; Wang, J.; Peng, W.; Zhang, L.; Xia, S.; et al. Multicolor Nitrogen-Doped Carbon Dots for Live Cell Imaging. J. Biomed. Nanotechnol. 2015, 11, 780–788. [Google Scholar] [CrossRef]
- Karakoçak, B.; Liang, J.; Kavadiya, S.; Berezin, M.; Biswas, P.; Ravi, N. Optimizing the synthesis of red-emissive nitrogen-doped carbon dots for use in bioimaging. ACS Appl. Nano Mater. 2018, 1, 3682–3692. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, K.; Zhao, L.; Li, C.; Bu, W.; Shen, Y.; Gu, Z.; Chang, B.; Zheng, C.; Lin, C.; et al. Aspirin-based carbon dots, a good biocompatibility of material applied for bioimaging and anti-inflammation. ACS Appl. Mater. Interfaces 2016, 8, 32706–32716. [Google Scholar] [CrossRef]
- Yao, H.; Li, J.; Song, Y.; Zhao, H.; Wei, Z.; Li, X.; Jin, Y.; Yang, B.; Jiang, J. Synthesis of ginsenoside Re-based carbon dots applied for bioimaging and effective inhibition of cancer cells. Int. J. Nanomed. 2018, 13, 6249–6264. [Google Scholar] [CrossRef]
- Niu, N.; Ma, Z.; He, F.; Li, S.; Li, J.; Liu, S.; Yang, P. Preparation of carbon dots for cellular imaging by the molecular aggregation of cellulolytic enzyme lignin. Langmuir 2017, 33, 5786–5795. [Google Scholar] [CrossRef]
- Tripathi, K.; Tran, T.; Tung, T.; Losic, D.; Kim, T. Water soluble Fluorescent Carbon Nano Dots from Bio-Source for Cells Imaging. J. Nanomater. 2016, 2017. [Google Scholar] [CrossRef]
- He, G.; Xu, M.; Mengjun, S.; Li, X.; Yang, Z.; Zhang, L.; Su, Y.; Hu, N.; Zhang, Y. Rapid solid-phase microwave synthesis of highly photoluminescent nitrogen-doped carbon dots for Fe(3+) detection and cellular bioimaging. Nanotechnology 2016, 27, 395706. [Google Scholar] [CrossRef]
- Emam, A.; Loutfy, S.; Mostafa, A.; Awad, H.; Mohamed, M. Cyto-toxicity, biocompatibility and cellular response of carbon dots–plasmonic based nano-hybrids for bioimaging. RSC Adv. 2017, 7, 23502–23514. [Google Scholar] [CrossRef]
- Li, L.; Lu, C.; Li, S.; Liu, S.; Wang, L.; Cai, W.; Xu, W.; Yang, X.; Liu, Y.; Zhang, R. A high-yield and versatile method for the synthesis of carbon dots for bioimaging applications. J. Mater. Chem. B 2017, 5, 1935–1942. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Lai, J.; Peng, X.; Hu, Y.; Gu, W.; Ye, L. Carbon dots with red-shifted photoluminescence by fluorine doping for optical bio-imaging. Carbon 2018, 128, 78–85. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Sun, Y.; Fan, J.; Li, X.; Zhang, Z. Photoluminescent lignin hybridized carbon quantum dots composites for bioimaging applications. Int. J. Biol. Macromol. 2019, 122, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Li, Y.; Zhu, R.; Shan, D.; Fan, Y.; Zhang, X. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sens. Actuators B 2015, 218, 229–236. [Google Scholar] [CrossRef]
- Zou, W.; Ji, Y.; Wang, X.; Zhao, Q.; Zhang, J.; Shao, Q.; Liu, J.; Wang, F.; Wang, Y. Insecticide as a precursor to prepare highly bright carbon dots for patterns printing and bioimaging: A new pathway for making poison profitable. Chem. Eng. J. 2016, 294, 323–332. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Rok Lee, Y. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid. Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, H.; Nanda, K. Boron-doped carbon nanoparticles: Size-independent color tunability from red to blue and bioimaging applications. Carbon 2016, 96, 166–173. [Google Scholar] [CrossRef]
- Puvvada, N.; Kumar, B.; Konar, S.; Kalita, H.; Mandal, M.; Mahanty, A.P. Synthesis of biocompatible multicolor luminescent carbon dots for bioimaging applications. Sci. Technol. Adv. Mater. 2012, 13, 045008. [Google Scholar] [CrossRef]
- Yang, C.; Thomsen, R.; Ogaki, R.; Kjems, J.; Teo, B. Ultrastable green fluorescence carbon dots with a high quantum yield for bioimaging and use as theranostic carriers. J. Mater. Chem. B 2015, 3, 4577–4584. [Google Scholar] [CrossRef]
- Zhang, M.; Chi, C.; Yuan, P.; Su, Y.; Shao, M.; Zhou, N. A hydrothermal route to multicolor luminescent carbon dots from adenosine disodium triphosphate for bioimaging. Mater. Sci. Eng. C 2017, 76, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, H.; Guo, C.; Zhai, Y.; Yang, J.; Yuan, J. A rapid microwave synthesis of green-emissive carbon dots with solid-state fluorescence and pH-sensitive properties. R. Soc. Open Sci. 2018, 5, 180245. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Zhang, C. Polyethyleneimine-functionalized fluorescent carbon dots: Water stability, ph sensing, and cellular imaging. ChemNanoMat 2015, 1, 122–127. [Google Scholar] [CrossRef]
- Chandra, S.; Das, P.; Bag, S.; Laha, D.; Pramanik, P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale 2011, 3, 1533–1540. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Zhang, H.; Liu, C.; Zhang, Y. Highly Luminescent Organosilane-Functionalized Carbon Dots. Adv. Funct. Mater. 2011, 21, 1027–1031. [Google Scholar] [CrossRef]
- Loukanov, A.; Mladenova, P.; Toshev, S.; Karailiev, A.; Ustinovich, E.; Nakabayashi, S. Real time monitoring and quantification of uptake carbon nanodots in eukaryotic cells. Microsc. Res. Tech. 2018, 81, 1541–1547. [Google Scholar] [CrossRef]
- Shakhov, A.; Astafiev, A.; Osychenko, A.; Syrchina, M.; Nadtochenko, V. Live cell bioimaging with carbon dots produced in situ by femtosecond laser from intracellular material. bioRxiv 2019. [Google Scholar] [CrossRef]
- Jung, Y.; Shin, E.; Kim, B. Cell nucleus-targeting zwitterionic carbon dots. Sci. Rep. 2015, 5, 18807. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, W.; Qiu, L.; Jiang, X.; Zuo, D.; Wang, D.; Yang, L. One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale 2015, 7, 6104–6113. [Google Scholar] [CrossRef]
- Hua, X.; Bao, Y.; Wu, F. Fluorescent carbon quantum dots with intrinsic nucleolus-targeting capability for nucleolus imaging and enhanced cytosolic and nuclear drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 10664–10677. [Google Scholar] [CrossRef]
- He, H.; Wang, Z.; Cheng, T.; Liu, X.; Wang, X.; Wang, J.; Ren, H.; Sun, Y.; Song, Y.; Yang, J.; et al. Visible and near-infrared dual-emission carbogenic small molecular complex with high rna selectivity and renal clearance for nucleolus and tumor imaging. ACS Appl. Mater. Interfaces 2016, 8, 28529–28537. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Wu, X. One-pot green synthesis of water-soluble carbon nanodots with multicolor photoluminescence from polyethylene glycol. J. Mater. Chem. B 2014, 2, 3937–3945. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, Y.; Wei, J.; Li, J.; Long, T.; Liu, Z. Optically active blue-emitting carbon dots to specifically target the Golgi apparatus. RSC Adv. 2017, 7, 49931–49936. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wu, H.; Song, X.; Guo, X.; Zhang, D.; Ma, X.; Tan, M. A mitochondria-targeted fluorescent probe based on TPP-conjugated carbon dots for both one- and two-photon fluorescence cell imaging. RSC Adv. 2014, 4, 49960–49963. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Teng, X.; Yan, M.; Bi, H.; Morais, P.C. Mitochondria-targeting nanoplatform with fluorescent carbon dots for long time imaging and magnetic field-enhanced cellular uptake. ACS Appl. Mater. Interfaces 2015, 7, 10201–10212. [Google Scholar] [CrossRef]
- Du, F.; Min, Y.; Zeng, F.; Yu, C.; Wu, S. A Targeted and FRET-based ratiometric fluorescent nanoprobe for imaging mitochondrial hydrogen peroxide in living cells. Small 2014, 10, 964–972. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Hu, C.; Yu, C.; Wu, S. Preparation of a mitochondria-targeted and no-releasing nanoplatform and its enhanced pro-apoptotic effect on cancer cells. Small 2014, 10, 3750–3760. [Google Scholar] [CrossRef]
- Jiang, X.; Zong, S.; Chen, C.; Zhang, Y.; Wang, Z.; Cui, Y. Gold–carbon dots for the intracellular imaging of cancer-derived exosomes. Nanotechnology 2018, 29, 175701. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gong, X.; Nan, M.; Liu, Y.; Shuang, S.; Dong, C. Comparative study for N and S doped carbon dots: Synthesis, characterization and applications for Fe3+ probe and cellular imaging. Anal. Chim. Acta 2015, 898, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Weng, B.; Wang, B.; Li, C. Facile synthesis of nitrogen and sulfur codoped carbon dots and application for Fe(III) ions detection and cell imaging. Sens. Actuators B 2016, 223, 689–696. [Google Scholar] [CrossRef]
- Kaur, H.; Raj, P.; Sharma, H.; Verma, M.; Singh, N.; Kaur, N. Highly selective and sensitive fluorescence sensing of nanomolar Zn2+ ions in aqueous medium using Calix[4]arene passivated carbon quantum dots based on fluorescence enhancement: Real-time monitoring and intracellular investigation. Anal. Chim. Acta 2018, 1009, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yan, F.; Luo, Y.; Ye, Q.; Zhou, S.; Chen, L. Amphiphilic carbon dots for sensitive detection, intracellular imaging of Al3+. Anal. Chim. Acta 2017, 953, 63–70. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Zhang, J.; Jia, J.; Zhao, D.; Fan, Y. Phenanthroline-derivative functionalized carbon dots for highly selective and sensitive detection of Cu2+ and S2− and imaging inside live cells. Nanomaterials 2018, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Misra, R. Mechanism of intracellular detection of glucose through nonenzymatic and boronic acid functionalized carbon dots. J. Biomed. Mater. Res. A 2015, 103, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Tian, Y.; Wang, Y.; Yang, W. Carbon-dot-based nanosensors for the detection of intracellular redox state. Adv. Mater. 2015, 27, 7156–7160. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Han, M.; Yuan, S.; Guan, W.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. N,S codoped carbon dots as a stable bio-imaging probe for detection of intracellular temperature and tetracycline. J. Mater. Chem. B 2017, 5, 3293–3299. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.; Wang, Y.; Froning, J.; Cepe, K.; Rogach, A.; Zbořil, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef]
- Shangguan, J.; He, D.; He, X.; Wang, K.; Xu, F.; Liu, J.; Tang, J.; Yang, X.; Huang, J. Label-free carbon-dots-based ratiometric fluorescence ph nanoprobes for intracellular pH sensing. Anal. Chem. 2016, 88, 7837–7843. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Singh, N. Biocompatible fluorescent carbon dots for ratiometric intracellular ph sensing. ChemistrySelect 2017, 2, 5723–5728. [Google Scholar] [CrossRef]
- Zholobak, N.; Popov, A.; Shcherbakov, A.; Popova, N.; Guzyk, M.; Antonovich, V.; Yegorova, A.; Scrypynets, Y.; Leonenko, I.; Baranchikov, A.; et al. Facile fabrication of luminescent organic dots by thermolysis of citric acid in urea melt, and their use for cell staining and polyelectrolyte microcapsule labelling. Beilstein J. Nanotechnol. 2016, 7, 1905–1917. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Maity, A.; Nandi, S.; Stepensky, D.; Jelinek, R. Imaging cancer cells expressing the folate receptor with carbon dots produced from folic acid. ChemBioChem 2016, 17, 614–619. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, B.; Zou, S.; Duan, B.; Chang, C.; Yang, B.; Zhou, X.; Zhang, L. Facile construction of carbon dots via acid catalytic hydrothermal method and their application for target imaging of cancer cells. Nano Res. 2016, 9, 214–223. [Google Scholar] [CrossRef]
- Motaghi, H.; Mehrgardi, M.; Bouvet, P. Carbon dots-AS1411 aptamer nanoconjugate for ultrasensitive spectrofluorometric detection of cancer cells. Sci. Rep. 2017, 7, 10513. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Liu, Y.; Lee, S. Carbon dots for bioimaging and biosensing applications. In Carbon-Based Nanosensor Technology; Kranz, C., Ed.; Springer: Cham, Switzerland, 2017; Volume 17. [Google Scholar] [CrossRef]
- Yang, S.; Cao, L.; Luo, P.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.; Liu, Y.; Qi, G.; Sun, Y. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef]
- Kuo, T.; Sung, S.; Hsu, C.; Chang, C.; Chiu, T.; Hu, C. One-pot green hydrothermal synthesis of fluorescent nitrogen-doped carbon nanodots for in vivo bioimaging. Anal. Bioanal. Chem. 2016, 408, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, S.; Liao, Y.; Li, S.; Ge, J.; Tao, F.; Huo, Q.; Zhang, Y.; Zhao, Z. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surf. B 2019, 174, 384–392. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Zhang, Z.; Yan, L.; Guo, L.; Niu, G.; Zhang, J.; Zhao, J.; Zhang, H.; Wang, P.; et al. Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy. Nano Res. 2017, 10, 3113–3123. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Liu, W.; Wang, X.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Te-containing carbon dots for fluorescence imaging of superoxide anion in mice during acute strenuous exercise or emotional changes. Chem. Sci. 2018, 9, 721–727. [Google Scholar] [CrossRef]
- Wei, X.; Xu, Y.; Li, Y.; Yin, X.; He, X. Ultrafast synthesis of nitrogen-doped carbon dots via neutralization heat for bioimaging and sensing applications. RSC Adv. 2014, 4, 44504–44508. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Fang, Y.; Xu, Y.; Wei, X.; Yin, X. Carbon quantum dots for zebrafish fluorescence imaging. Sci. Rep. 2015, 5, 11835. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.; Blackwelder, P.; Leblanc, R. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: A zebrafish model study. Colloids Surf. B 2016, 145, 251–256. [Google Scholar] [CrossRef]
- Wei, X.; Li, L.; Liu, J.; Yu, L.; Li, H.; Cheng, F.; Yi, X.; He, J.; Li, B. Green Synthesis of Fluorescent Carbon Dots from Gynostemma for Bioimaging and Antioxidant in Zebrafish. ACS Appl. Mater. Interfaces 2019, 11, 9832–9840. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Skromne, I.; Peng, Z.; Dallman, J.; Al-Youbi, A.; Bashammakh, A.; El-Shahawi, M.; Leblanc, R. “Dark” carbon dots specifically “light-up” calcified zebrafish bones. J. Mater. Chem. B 2016, 4, 7398–7405. [Google Scholar] [CrossRef]
- Saxena, M.; Sonkar, S.; Sarkar, S. Water soluble nanocarbons arrest the growth of mosquitoes. RSC Adv. 2013, 3, 22504–22508. [Google Scholar] [CrossRef]




| Source | Synthesis Method 1 | λex/λem (nm) | Type of cel$l s | Reference |
|---|---|---|---|---|
| Histidine | M: 200 °C, 1 h | 360/439 | AD-293 | [6] |
| Curcumin and polyethyleneimine | S: 200 °C, 12 h | 340/450 | A549, NIH 3T3 and HCT-15 | [31] |
| Citrus sinensis or Citrus limon | P: 180 °C, 2 h | 365/455 or 330/390–435 (dual emission) | A549, HeLa, MDA-MB-231, and HEK-293 | [15] |
| Apple seeds | P: 250 °C, 3 h | 290–340/380–415 (dual emission) | A549, HeLa, MDA-MB-231 and HEK-293 | [14] |
| Human fingernails and sulfuric acid | M:400 W, 2 min | 320/380 | A549, HeLa, MDA-MB-231, and HEK-293 | [16] |
| Human fingernails | P: 200 °C, 3 h | 330–370/380–450 (dual emission) | A549, HeLa, MDA-MB-231 and HEK-293 | [17] |
| Glucose and glycine | S: 180 °C, 6 h | 370/475 | Sh-y5y and MTEC1 | [44] |
| Citric acid and ethylenediamine | M:1100 W, 88 s | 540/600 | Retinal epithelial, lens epithelial and CHO cells | [45] |
| Aspirin and hydrazine | M:500 W, 8 min | 360/432 | RAW246.7, HeLa, KB, and BMSC | [46] |
| Cotton | P: 300 °C, 2 h | 330/460 | A193 | [18] |
| L-citrulline | S: 220 °C, 12 h | 350/450 | HeLa | [19] |
| Coriander leaves | S: 240 °C, 4 h | 320/400 | A549, L-132 | [20] |
| Ginsenoside Re, citric acid, and ethylenediamine | S: 200 °C, 10 h | 360/460 | A375 | [47] |
| Cellulolytic enzyme lignin | 6 h stirring in ethanol | 280/390 | HeLa | [48] |
| Kidney beans | P: 450 °C, 2 h | 340/460 | HeLa | [49] |
| Citric acid and tris(hydroxymethyl) methylaminomethane | S: 250 °C, 6 h | 330/420 | MCF-7 | [22] |
| L-glutamic acid and silica gel | M:640 W, 5 min | 370/450 | BT-474 | [50] |
| Citric acid and ethylenediamine | M:600 W, 13 min | 366/488 | HepG2 | [51] |
| Citric acid, sodium nitrate, and potassium nitrate | P: 220 °C, 6 h | 330/440 | HeLa | [52] |
| Citric acid, urea, and sodium fluoride | M:750 W, 5 min | 530/600 | C6 glioma cells | [53] |
| Citric acid, ethylenediamine, and alkali lignin | S: 150 °C, 4 h | 377/454 | HeLa | [54] |
| Alanine and ethylenediamine | S: 200 °C, 6 h | 320/390 | MCF-7 | [55] |
| 3-Bromophenol | S: 170 °C, 8 h | 318/430 | HeLa | [56] |
| Peach extract and ammonia | S: 180 °C, 5 h | 325/404 | MDA-MB-231 | [57] |
| Boric acid and sucrose | S: 180 °C, 10 h | 365/450 | HeLa | [58] |
| Dextrin and sulfuric acid | M:800 W, 2.5 min | 440/486 | MDA-MB-231 | [59] |
| Cyclodextrin, sulfuric acid, and ethylenediamine | stirring 2 h at 90 °C | 390/510 | H1299 | [60] |
| Carrot juice | S: 160 °C, 6 h | 360/442 | HaCaT | [21] |
| Adenosine-5′-triphosphate | S: 180 °C, 10 h | 340/440 | A549 | [61] |
| Phthalic acid and triethylenediamine | M:700 W, 1 min | 360/500 | HeLa | [62] |
| Citric acid and polyethyleneimine | S: 180 °C, 20 h | 420/460 | CAL-27 | [63] |
| Polyethyleneimine and nitric acid | reflux at 120 °C for 72 h | 320/420 | HeLa | [23] |
| Sucrose and phosphoric acid | M:100 W, 3 min and 40 s | 225/453 | red blood cells | [64] |
| N-(β-aminoethyl)-γ-aminopropyl methyl dimethoxy silane and citric acid | heating at 240 °C for 1 min | 340/450 | BGC823 | [65] |
| Glycerol and 4,7,10-trioxa-1,13-tridecanediamine | M:700 W, 10 min | 340/450 | HepG-2 | [27] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging Applications of Carbon Nanodots: A Review. C 2019, 5, 19. https://doi.org/10.3390/c5020019
Kasouni A, Chatzimitakos T, Stalikas C. Bioimaging Applications of Carbon Nanodots: A Review. C. 2019; 5(2):19. https://doi.org/10.3390/c5020019
Chicago/Turabian StyleKasouni, Athanasia, Theodoros Chatzimitakos, and Constantine Stalikas. 2019. "Bioimaging Applications of Carbon Nanodots: A Review" C 5, no. 2: 19. https://doi.org/10.3390/c5020019
APA StyleKasouni, A., Chatzimitakos, T., & Stalikas, C. (2019). Bioimaging Applications of Carbon Nanodots: A Review. C, 5(2), 19. https://doi.org/10.3390/c5020019