Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation
Abstract
:1. Introduction
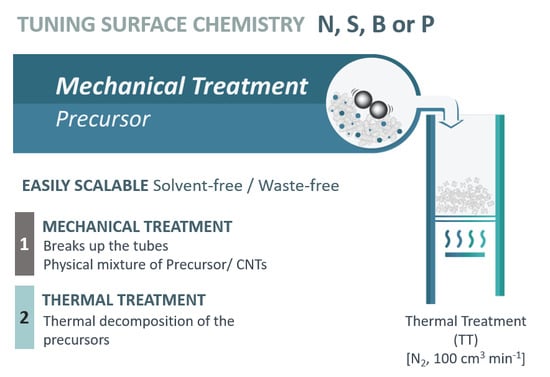
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Catalytic Wet Air Oxidation (CWAO) Experimental Procedure
2.4. Catalytic Wet Air Oxidation (CWAO) Analytical Techniques
3. Results and Discussion
3.1. Materials Characterization
3.2. Catalytic Activity in Catalytic Wet Air Oxidation (CWAO)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, V.S.; Mahajani, V.V.; Joshi, J.B. Wet Air Oxidation. Ind. Eng. Chem. Res. 1995, 34, 2–48. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Sahibzada, M.; Livingston, A.G.; Metcalfe, I.S.; Hellgardt, K. Wastewater treatment: Wet air oxidation as a precursor to biological treatment. Catal. Today 1999, 53, 93–106. [Google Scholar] [CrossRef]
- Kolaczkowski, S.T.; Plucinski, P.; Beltran, F.J.; Rivas, F.J.; McLurgh, D.B. Wet air oxidation: A review of process technologies and aspects in reactor design. Chem. Eng. J. 1999, 73, 143–160. [Google Scholar] [CrossRef]
- Luck, F. Wet air oxidation: Past, present and future. Catal. Today 1999, 53, 81–91. [Google Scholar] [CrossRef]
- Debellefontaine, H.; Foussard, J.N. Wet air oxidation for the treatment of industrial wastes. Chemical aspects, reactor design and industrial applications in Europe. Waste Manag. 25. [CrossRef]
- Stüber, F.; Font, J.; Fortuny, A.; Bengoa, C.; Eftaxias, A.; Fabregat, A. Carbon materials and catalytic wet air oxidation of organic pollutants in wastewater. Top. Catal. 2005, 33, 3–50. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Foger, K.; Akolekar, D.B.; Grocott, S.C. Wet Oxidation and Catalytic Wet Oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes: A review. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Cybulski, A. Catalytic Wet Air Oxidation: Are Monolithic Catalysts and Reactors Feasible? Ind. Eng. Chem. Res. 2007, 46, 4007–4033. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Castelo-Branco, I.M.; Quinta-Ferreira, R.M.; Levec, J. Catalytic studies in wet oxidation of effluents from formaldehyde industry. Chem. Eng. Sci. 2003, 58, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.T.; Quinta-Ferreira, R.M.; Levec, J. Catalytic and Noncatalytic Wet Oxidation of Formaldehyde. A Novel Kinetic Model. Ind. Eng. Chem. Res. 2003, 42, 5099–5108. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.T.; Marques, R.R.N.; Quinta-Ferreira, R.M. Catalysts based in cerium oxide for wet oxidation of acrylic acid in the prevention of environmental risks. Appl. Catal. B Environ. 2004, 47, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.T.; Oliveira, A.C.M.; Quinta-Ferreira, R.M. Catalytic wet oxidation of ethylene glycol: Kinetics of reaction on a Mn–Ce–O catalyst. Chem. Eng. Sci. 2004, 59, 5291–5299. [Google Scholar] [CrossRef]
- Oliviero, L.; Barbier, J.; Duprez, D.; Wahyu, H.; Ponton, J.W.; Metcalfe, I.S.; Mantzavinos, D. Wet air oxidation of aqueous solutions of maleic acid over Ru/CeO2 catalysts. Appl. Catal. B Environ. 2001, 35, 1–12. [Google Scholar] [CrossRef]
- Oliviero, L.; Wahyu, H.; Barbier, J., Jr.; Duprez, D.; Ponton, J.W.; Metcalfe, I.S.; Mantzavinos, D. Experimental and Predictive Approach for Determining Wet Air Oxidation Reaction Pathways in Synthetic Wastewaters. Chem. Eng. Res. Des. 2003, 81, 384–392. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Rodríguez, J.J. Catalytic wet air oxidation of phenol with modified activated carbons and Fe/activated carbon catalysts. Appl. Catal. B Environ. 2007, 76, 135–145. [Google Scholar] [CrossRef]
- Quintanilla, A.; Menéndez, N.; Tornero, J.; Casas, J.A.; Rodríguez, J.J. Surface modification of carbon-supported iron catalyst during the wet air oxidation of phenol: Influence on activity, selectivity and stability. Appl. Catal. B Environ. 2008, 81, 105–114. [Google Scholar] [CrossRef]
- Aguilar, C.; García, R.; Soto-Garrido, G.; Arriagada, R. Catalytic wet air oxidation of aqueous ammonia with activated carbon. Appl. Catal. B Environ. 2003, 46, 229–237. [Google Scholar] [CrossRef]
- Eftaxias, A.; Font, J.; Fortuny, A.; Fabregat, A.; Stüber, F. Kinetics of phenol oxidation in a trickle bed reactor over active carbon catalyst. J. Chem. Technol. Biotechnol. 2005, 80, 677–687. [Google Scholar] [CrossRef]
- Eftaxias, A.; Font, J.; Fortuny, A.; Fabregat, A.; Stüber, F. Catalytic wet air oxidation of phenol over active carbon catalyst: Global kinetic modelling using simulated annealing. Appl. Catal. B Environ. 2006, 67, 12–23. [Google Scholar] [CrossRef]
- Santiago, M.; Stüber, F.; Fortuny, A.; Fabregat, A.; Font, J. Modified activated carbons for catalytic wet air oxidation of phenol. Carbon 2005, 43, 2134–2145. [Google Scholar] [CrossRef]
- Suarez-Ojeda, M.E.; Stüber, F.; Fortuny, A.; Fabregat, A.; Carrera, J.; Font, J. Catalytic wet air oxidation of substituted phenols using activated carbon as catalyst. Appl. Catal. B Environ. 2005, 58, 105–114. [Google Scholar] [CrossRef]
- Cordero, T.; Rodríguez-Mirasol, J.; Bedia, J.; Gomis, S.; Yustos, P.; García-Ochoa, F.; Santos, A. Activated carbon as catalyst in wet oxidation of phenol: Effect of the oxidation reaction on the catalyst properties and stability. Appl. Catal. B Environ. 2008, 81, 122–131. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Hydrogen peroxide-promoted-CWAO of phenol with activated carbon. Appl. Catal. B Environ. 2010, 93, 339–345. [Google Scholar] [CrossRef]
- Fortuny, A.; Font, J.; Fabregat, A. Wet air oxidation of phenol using active carbon as catalyst. Appl. Catal. B Environ. 1998, 19, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.P.; Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B Environ. 2011, 104, 330–336. [Google Scholar] [CrossRef]
- Sun, X.; Wang, R.; Su, D. Research progress in metal-free carbon-based catalysts. Chin. J. Catal. 2013, 34, 508–523. [Google Scholar] [CrossRef]
- Rocha, R.P.; Silva, A.M.T.; Romero, S.M.M.; Pereira, M.F.R.; Figueiredo, J.L. The role of O- and S-containing surface groups on carbon nanotubes for the elimination of organic pollutants by catalytic wet air oxidation. Appl. Catal. B Environ. 2014, 147, 314–321. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Application of Nanocarbon Materials to Catalysis. In Nanotechnology in Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 37–56. [Google Scholar]
- Figueiredo, J.L.; Pereira, M.F.R. Carbon as Catalyst. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 177–217. [Google Scholar]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Mechanochemistry and sonochemistry: Concluding remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef]
- Ma, P.C.; Wang, S.Q.; Kim, J.K.; Tang, B.Z. In-situ amino functionalization of carbon nanotubes using ball milling. J. Nanosci. Nanotechnol. 2009, 9, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Yuhua, X.; Hao, C.; Jia, Q.; Liming, D. Nitrogen-doped graphene by ball-milling graphite with melamine for energy conversion and storage. 2D Mater. 2015, 2, 044001. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Jarrais, B.; Guedes, A.; Freire, C. Heteroatom-Doped Carbon Nanomaterials as Metal-Free Catalysts for the Reduction of 4-Nitrophenol. ChemistrySelect 2018, 3, 1737–1748. [Google Scholar] [CrossRef]
- Hu, C.; Liu, D.; Xiao, Y.; Dai, L. Functionalization of graphene materials by heteroatom-doping for energy conversion and storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 121–132. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Dai, L.; Baek, J.-B. Large-Scale Production of Edge-Selectively Functionalized Graphene Nanoplatelets via Ball Milling and Their Use as Metal-Free Electrocatalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shui, J.; Wang, J.; Wang, M.; Liu, H.-K.; Dou, S.X.; Jeon, I.-Y.; Seo, J.-M.; Baek, J.-B.; Dai, L. Sulfur–Graphene Nanostructured Cathodes via Ball-Milling for High-Performance Lithium–Sulfur Batteries. ACS Nano 2014, 8, 10920–10930. [Google Scholar] [CrossRef]
- Woodman, R.H.; Klotz, B.R.; Dowding, R.J. Evaluation of a dry ball-milling technique as a method for mixing boron carbide and carbon nanotube powders. Ceram. Int. 2005, 31, 765–768. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, Y.; Wang, L.; Li, N.; Chen, C.; Li, C.; Li, J.; Lv, H.; Kuang, L.; Tian, X. Nanoconfined phosphorus film coating on interconnected carbon nanotubes as ultrastable anodes for lithium ion batteries. J. Power Sources 2017, 356, 18–26. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Goncalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of carbon nanotubes by ball-milling to be used as ozonation catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef]
- Rocha, R.P.; Soares, O.S.G.P.; Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Different methodologies for synthesis of nitrogen doped carbon nanotubes and their use in catalytic wet air oxidation. Appl. Catal. A Gen. 2017, 548, 62–70. [Google Scholar] [CrossRef]
- Rocha, R.P.; Santos, D.F.M.; Soares, O.S.M.P.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Metal-Free Catalytic Wet Oxidation: From Powder to Structured Catalyst Using N-Doped Carbon Nanotubes. Top. Catal. 2018, 61, 1957–1966. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Loiseau, A.; Launois-Bernede, P.; Petit, P.; Roche, S.; Salvetat, J.-P. (Eds.) Understanding Carbon Nanotubes from Basics to Applications Series: Lecture Notes in Physics; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; Volume 677. [Google Scholar]
- Seetapan, N.; Limparyoon, N.; Kiatkamjornwong, S. Effect of fire retardant on flammability of acrylamide and 2-acrylamido-2-methylpropane sodium sulfonate copolymer composites. Polym. Degrad. Stab. 2011, 96, 1927–1933. [Google Scholar] [CrossRef]
- Piękoś, R.; Wesołowski, M.; Teodorczyk, J. Thermal Analysis of a Hydrated Silica–Sodium Thiosulfate–Sulfur System. J. Therm. Anal. Calorim. 2001, 66, 541. [Google Scholar] [CrossRef]
- Sekkina, M.A.; El-Shereafy, E.-S.; Mashaly, A.; El-Ashry, M. γ-Pyrolysis of crystalline sodium thiosulphate penta hydrate. J. Radioanal. Nucl. Chem. 1998, 237, 113–119. [Google Scholar] [CrossRef]
- de Jager, H.-J.; Prinsloo, L.C. The dehydration of phosphates monitored by DSC/TGA and in situ Raman spectroscopy. Thermochim. Acta 2001, 376, 187–196. [Google Scholar] [CrossRef]
- Jinlong, N.; Zhenxi, Z.; Dazong, J. Investigation of phase evolution during the formation of calcium potassium sodium orthophosphate. Mater. Chem. Phys. 2003, 78, 308–312. [Google Scholar] [CrossRef]
- Sevim, F.; Demir, F.; Bilen, M.; Okur, H. Kinetic analysis of thermal decomposition of boric acid from thermogravimetric data. Korean J. Chem. Eng. 2006, 23, 736–740. [Google Scholar] [CrossRef]
- Pankajavalli, R.; Anthonysamy, S.; Ananthasivan, K.; Vasudeva Rao, P.R. Vapour pressure and standard enthalpy of sublimation of H3BO3. J. Nucl. Mater. 2007, 362, 128–131. [Google Scholar] [CrossRef]
- Boehm, H.-P. Catalytic Properties of Nitrogen-Containing Carbons. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 219–265. [Google Scholar]
- Alexander, V.; Naumkin, A.K.-V.; Stephen, W.G.; Cedric, J.P. NIST Standard Reference Database 20, Version 3.4 (web version). Available online: https://srdata.nist.gov/xps/Version_his.aspx (accessed on 31 October 2018).
- Soria-Sánchez, M.; Maroto-Valiente, A.; Álvarez-Rodríguez, J.; Muñoz-Andrés, V.; Rodríguez-Ramos, I.; Guerrero-Ruíz, A. Carbon nanostrutured materials as direct catalysts for phenol oxidation in aqueous phase. Appl. Catal. B Environ. 2011, 104, 101–109. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zhu, W.; Wang, J.; Descorme, C. Catalytic activity, stability and structure of multi-walled carbon nanotubes in the wet air oxidation of phenol. Carbon 2008, 46, 445–452. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, W.; Li, X.; Wang, J.; Zhou, Y. Multi-walled carbon nanotubes (MWNTs) as an efficient catalyst for catalytic wet air oxidation of phenol. Catal. Commun. 2007, 8, 2059–2063. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Yang, H.; Sun, Y.; Liu, Y. Influence of the different oxidation treatment on the performance of multi-walled carbon nanotubes in the catalytic wet air oxidation of phenol. J. Hazard. Mater. 2012, 233–234, 18–24. [Google Scholar] [CrossRef]
- Wang, J.; Fu, W.; He, X.; Yang, S.; Zhu, W. Catalytic wet air oxidation of phenol with functionalized carbon materials as catalysts: Reaction mechanism and pathway. J. Environ. Sci. 2014, 26, 1741–1749. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Yang, H.; Wan, J. Catalytic wet air oxidation of phenol, nitrobenzene and aniline over the multi-walled carbon nanotubes (MWCNTs) as catalysts. Front. Environ. Sci. Eng. 2015, 9, 436–443. [Google Scholar] [CrossRef]
- Restivo, J.; Rocha, R.P.; Silva, A.M.T.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic performance of heteroatom-modified carbon nanotubes in advanced oxidation processes. Cuihua Xuebao Chin. J. Catal. 2014, 35, 896–905. [Google Scholar] [CrossRef]
- Milone, C.; Shahul Hameed, A.R.; Piperopoulos, E.; Santangelo, S.; Lanza, M.; Galvagno, S. Catalytic Wet Air Oxidation of p-Coumaric Acid over Carbon Nanotubes and Activated Carbon. Ind. Eng. Chem. Res. 2011, 50, 9043–9053. [Google Scholar] [CrossRef]
- Xing, B.; Chen, H.; Zhang, X. Efficient degradation of organic phosphorus in glyphosate wastewater by catalytic wet oxidation using modified activated carbon as a catalyst. Environ. Technol. 2018, 39, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Wet Air Oxidation of Aniline Using Carbon Foams and Fibers Enriched with Nitrogen. Sep. Sci. Technol. 2010, 45, 1546–1554. [Google Scholar] [CrossRef]
- Rocha, R.P.; Restivo, J.; Sousa, J.P.S.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Nitrogen-doped carbon xerogels as catalysts for advanced oxidation processes. Catal. Today 2015, 241, 73–79. [Google Scholar] [CrossRef]
- Chen, H.; Yang, G.; Feng, Y.; Shi, C.; Xu, S.; Cao, W.; Zhang, X. Biodegradability enhancement of coking wastewater by catalytic wet air oxidation using aminated activated carbon as catalyst. Chem. Eng. J. 2012, 198–199, 45–51. [Google Scholar] [CrossRef]
- Dong, F.; Cai, Y.; Liu, C.; Liu, J.; Qiao, J. Heteroatom (B, N and P) doped porous graphene foams for efficient oxygen reduction reaction electrocatalysis. Int. J. Hydrog. Energy 2018, 43, 12661–12670. [Google Scholar] [CrossRef]
- He, W.; Xue, P.; Du, H.; Xu, L.; Pang, M.; Gao, X.; Yu, J.; Zhang, Z.; Huang, T. A facile method prepared nitrogen and boron doped carbon nano-tube based catalysts for oxygen reduction. Int. J. Hydrog. Energy 2017, 42, 4123–4132. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, G.-F.; Li, D.-F.; Zhang, M.; Pan, A.-L.; Huang, W.-Q. Facile synthesis and superior photocatalytic and electrocatalytic performances of porous B-doped g-C3N4 nanosheets. J. Mater. Sci. Technol. 2018, 34, 2515–2520. [Google Scholar] [CrossRef]
- Serp, P.; Machado, B. CHAPTER 6 Doped Nanostructured Carbon Materials as Catalysts. In Nanostructured Carbon Materials for Catalysis; The Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Serp, P.; Machado, B. CHAPTER 5 Nanostructured Carbon Materials as Catalysts. In Nanostructured Carbon Materials for Catalysis; The Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. P-, B- and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catal. Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]






| Sample. | Description | Precursor Name | Formula | Thermal Treatment Temperature (°C) |
|---|---|---|---|---|
| CNT-O | Pristine Material | - | - | - |
| CNT-N | N-doped CNTs | Melamine | C3H6N6 | 600 |
| CNT-S | S-doped CNTs | Sodium thiosulfate | Na2S2O3 | 900 |
| CNT-P | P-doped CNTs | Sodium dihydrogen phosphate | NaH2PO4 | 300 |
| CNT-B | B-doped CNTs | Boric acid | H3BO3 | 200 |
| Sample | SBET (m2 g−1) | Vp (cm3 g−1) |
|---|---|---|
| CNT-O | 192 | 0.452 |
| CNT-N | 211 | 0.515 |
| CNT-S | 172 | 0.356 |
| CNT-P | 93 | 0.230 |
| CNT-B | 45 | 0.077 |
| Sample | EA (% wt.) | XPS (% wt.) | TGA (% wt.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | H | S | O | C | O | S/P/B | Na | Volatiles | Cfixed | Ash | |
| CNT-O | 97.5 | 0.0 | 0.1 | 0.0 | 1.2 | 8.7 | 91.1 | 0.2 | ||||
| CNT-N | 93.3 | 3.5 | 1.3 | 0.0 | 1.4 | 8.6 | 90.8 | 0.6 | ||||
| CNT-S | 54.2 | 0.0 | 0.5 | 9.2 | 26.5 | 59.7 | 22.9 | 7.0 (S) | 10.4 | 29.3 | 21.3 | 49.4 |
| CNT-P | 41.4 | 0.0 | 0.3 | 0.0 | 28.0 | 78.6 | 13.5 | 4.5 (P) | 3.4 | 8.2 | 39.5 | 52.3 |
| CNT-B | 33.7 | 0.0 | 2.6 | 0.0 | 44.1 | 31.4 | 49.9 | 18.7 (B) | 5.3 | 32.0 | 62.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, O.S.G.P.; Rocha, R.P.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation. C 2019, 5, 30. https://doi.org/10.3390/c5020030
Soares OSGP, Rocha RP, Órfão JJM, Pereira MFR, Figueiredo JL. Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation. C. 2019; 5(2):30. https://doi.org/10.3390/c5020030
Chicago/Turabian StyleSoares, Olívia Salomé G. P., Raquel P. Rocha, José J. M. Órfão, Manuel Fernando R. Pereira, and José L. Figueiredo. 2019. "Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation" C 5, no. 2: 30. https://doi.org/10.3390/c5020030
APA StyleSoares, O. S. G. P., Rocha, R. P., Órfão, J. J. M., Pereira, M. F. R., & Figueiredo, J. L. (2019). Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation. C, 5(2), 30. https://doi.org/10.3390/c5020030