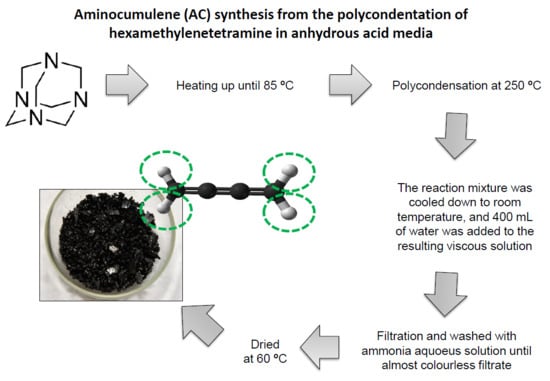
3.1. Synthesis of Soluble and Insoluble AC
The experiments showed that when using concentrated sulfuric acid (95% H2SO4 and 5% water) as a reaction medium instead of 5 wt% oleum (95% H2SO4 and 5% SO3), the AC yield (both soluble and insoluble) decreases remarkably. Thus, the absence of water during the polycondensation of HMTA plays an important role for enhancing the AC yield. As it was mentioned previously, the reaction of HMTA polycondensation in anhydrous sulfuric acid or oleum was accomplished with some evolution of gaseous sulfur dioxide and foaming at the reaction mixture. Due to the foaming formation, the maximal loading of the reactor was limited to not more than 45–60 mL of oleum and 30 g HMTA per 2 L of reactor volume. Thus, yield of the process per volume of the reactor was low. We tried to increase the yield by gradually loading the reactor simultaneously with small volumes of reagents (HMTA and 5 wt% oleum) to the “seed” of the reaction mixture by keeping the temperature near 150 °C. Under such conditions, the reaction proceeded calmly, and there was no vigorous foaming formation; nonetheless, the AC yield decreased sharply compared to a single synthesis in the “flash” mode.
Taking into account the complexity of carbyne-like compounds synthesis according to the reported literature [
11], we described here a novel, facile chemical procedure using HMTA as a precursor for AC synthesis. As is known, HMTA is usually obtained by the interaction of formaldehyde with ammonia. This reaction is reversible in aqueous media, and when the acid is added, the equilibrium in the aqueous solution is displaced to the opposite direction due to the protonation of ammonia under acidic conditions. However, the protonation of the nitrogen atoms in HMTA under an anhydrous medium at strongly acidic pH might lead to an increase of the mobility of methylene hydrogen atoms due to the electron density shifting to positively charged protonated nitrogen atoms, thereby prompting apparently unknown chemical reactions.
Formally, the overall reaction (1) could be assumed to explain the synthesis of AC:
Based on the standard thermodynamic data [
13] for reagents and products in the hypothetical chemical reaction (1), the standard enthalpy of formation in (1) corresponds to a −632.4 kJ/reaction formula when carbon is produced in the form of graphite, and a −621.6 kJ/reaction formula when carbon is produced in diamond form. Obviously, the reserve of Gibbs free energy in this reaction is large enough to enable the formation of less stable forms of carbon, and therefore our process is thermodynamically possible. In this regard, our experiments show that in anhydrous sulfuric acid medium, HMTA is capable of undergoing polycondensation with the formation of carbon-like species. Furthermore, upon the stepwise addition of HMTA into 5 wt% oleum solution under vigorous stirring and cooling conditions with temperatures not higher than 50–60 °C, a colorless solution is formed probably associated to HMTA hydrogen sulfate, which crystallizes upon cooling up to rt. However, if the reaction temperature is raised up to 85–90 °C, an exothermic reaction is initiated, and the temperature increases spontaneously up to 240–250 °C. Under our experimental conditions described in
Section 2.2, a black, brown, thin layer of resin is obtained, which is almost a solid at rt. This product dissolves well in water, although depending on the temperature range, it may contain a certain amount of insoluble fraction. Moreover, if after an exothermic “flash” mode condition the reaction mixture is kept for 1–2 h at 180–200 °C, the polycondensation proceeds far beyond reaching an insoluble black carbon-like product, as mentioned in
Section 2.3.
A tentative mechanism is proposed for the synthesis of AC from the polycondensation of HMTA. It can be assumed that during the protonation of nitrogen atoms in HMTA under anhydrous medium at strongly acidic pH, the C–N bond can be broken, whereby the protonated nitrogen behaves as an electronegative group. When the protonation of two nitrogen atoms adjacent to one carbon atom takes place, which is possible in strongly acidic medium, the mobility of methylene hydrogens increases, and therefore, they can also be split off. Accordingly, the entire process can be explained by the following mechanism.
Where step (2) displays the protonation of HMTA by using sulfuric acid: (herein >N-CH
2-N< is a fragment of the cyclic structure of HMTA), and step (3) represents the cleavage of the methylene hydrogen and ammonium group in order to form the carbene species. In step (4), the carbene dimerization and further protonation of the dimer takes place in the presence of sulfuric acid, as described in step (5), followed by step (6), which corresponds to the cleavage of the ammonium group from the protonated dimer to form the carbene, with the carbene dimerization finally occurring in step (7). Further chemical reactions involving step (7) can be repeatedly run via the protonation of nitrogen atoms, removal of ammonium ions, and dimerization of the carbene formed in order to increase the length of the cumulene chain. Moreover, carbene species may likely react with C=C bonds with the formation of branched polymeric structures.
When the reaction mixture is diluted with water, the acid-catalyzed hydrolysis of unreacted fragments >N-CH
2-N< results in the formation of protonated amino groups, according to reaction (8):
In this regard, the resulting oligomeric products dissolve in water at acidic pH due to the protonation of amino groups. Nonetheless, the addition of ammonia, according to chemical reaction (9) leads to deprotonation of amino groups, and therefore the resulting AC loses its solubility in water:
when diluting the acidic reaction mixture with water, an adverse reaction may also occur, with the participation of the tautomers, according to reaction (10).
As a result of the above reaction in an acid medium, nitrogen can be replaced with oxygen to form an aldehyde group. However, in aqueous ammonia medium, reaction (10) can be reversed, as described in reaction (11):
For that reason, AC dissolves first in water containing sulfuric acid, and then is treated with ammonia to precipitate the AC, whereby the product can contain oxygen groups, and therefore its amount in at% will depend on the synthesis conditions. Accordingly, the final product from the result of HMTA polycondensation can be referred as “aminocumulene”. Obviously, this is a technical name, similar to the product itself, which is made of a mixture of oligomers of linear and/or branched structures. The aforementioned proposed mechanism explains why the yield of the HMTA polycondensation is higher when performing the reaction using the “flash mode” rather than under the gradual addition of reagents to the reaction mixture. In this regard, the former synthetic approach will have a greatest concentration of carbene species, thereby prompting the most favorable conditions for its dimerization. Furthermore, we speculate that the amino groups are adjacent to the double bond, i.e., H2N-CH=C=, although one should consider the possibility of the tautomerization of such a structure through the transition of the hydrogen atom from a nitrogen to a carbon atom, as described in chemical reaction (10).
3.2. Physicochemical Properties of the AC
The AC was characterized through SEM, elemental analysis, spectral analysis, and surface chemistry analysis.
Figure 1 shows the SEM image at two magnifications of the cleavage surface of the soluble AC, confirming a smooth, homogeneous surface.
Table 1 compiles the elemental semiquantitative analysis obtained from the energy-dispersive analysis, demonstrating the presence of a high nitrogen at% as expected, but also the presence of oxygen as inferred from chemical reaction (10) during the AC synthesis. Sulfur is present in a trace amount (not more than 1 wt%). Elemental analysis is also in agreement with the XPS data depicted in
Figure 2. Carbon, nitrogen, and oxygen core energy levels of AC confirmed the presence of the above elements. The C 1s core level spectrum was deconvoluted to various contributions. The intense peak at 284.6 eV (39.21 at%) was assigned to carbon in sp2 configurations, the peak at 285.76 eV (22.6 at%) was linked to C–O, C–H, and or H
2N–C bonds (phenolic, alkoxy, ether and/or amines), and the contribution featuring at 287.1 eV (8.31 at%) was assigned to a CH=O bond (carbonyl and/or aldehyde groups). The O 1s core level signal was decomposed in contributions at 530.8 eV (7.0 at%), and was attributed to oxide or/and hydroxide, 532.26 eV (4.55 at%) was assigned to C=O bonds (aldehyde/carbonyl), and 533.74 eV (0.34 at%) was ascribed to a C–O bond (phenol/epoxy/ether). With regard to the N 1s core energy level, the deconvolution provided the features at a binding energy of 398.39 eV (7.91 at%) linked to the presence of =CH-NH
2 (amine groups), while 399.97 eV (8.06 at%) linked to the presence of C–N–C and was attributed to pyrrolic and/or pyridinic groups, and 401.26 eV (2.03 eV) can be attributed to a C–N bond, such as for example in quaternary nitrogen species. In general, the observed spectra do not contradict the expected structure of the HMTA polycondensation products. Nonetheless, it cannot be ruled out that other compounds are present in the reaction products, so that the assignment of spectral data cannot be considered to be utterly unambiguous.
With the aim of comparing our results to those in the literature, it is known that the oxygen analogue vinyl alcohol is unstable, because it isomerizes to acetaldehyde. Saebo and Radom [
14] performed a quantum mechanical calculation of the vinylamine molecule, which is an analogous moiety of the AC compound synthesized in this work, and predicted the non-planarity of vinylamine with a pyramidal amino group configuration. Nonetheless, vinylamine was prepared by gas-phase pyrolysis via aliphatic amines, and the half-life of this molecule was about 10 min [
15,
16]. Additional work performed by Pillsbury and Drucker [
17] reported that vinylamine is poorly stable at room temperature, exhibiting a half-life between 2–20 min. Our XPS data are inconsistent with regard to the stability of vinylamine, and hence, we confirm the existence of enamine tautomeric forms. Accordingly, the tautomeric equilibrium (see the chemical reaction number 10) is more likely shifted toward the enamine form, unlike the analogous vinyl alcohol moiety. Thus, the presence of the amine group adjacent to the double carbon–carbon bond upon the structures can be presumably proposed in this work through the polycondensation of HMTA.
Figure 3A depicts the UV-Vis absorption spectrum of the AC in 1.0 M of acetic acid, showing a broad band with a maximum peak at 465 nm. In general, the observed spectrum is similar to the absorption spectra of the carbyne-like substances given and synthesized in [
18,
19]. However, the exact correspondence of the UV-Vis spectra cannot be expected, because of all the above molecules are different, connected only by the presence presumably of a carbyne-like fragment of the carbon chain. Nonetheless, it is well established that the factors that govern the UV-Vis spectrum of a cumulene derivative compound include the structure of the [n]cumulene, its length, and the nature of the endcapping groups with mesomeric or inductive properties. In a recent review, Januszewski and Tykwinski [
10] stated that even though the maximum electronic absorption is obtained in the UV region (below 400 nm and dependent on the molecular length), the presence of other electronic absorption peaks is found in the visible region ranging between 420–670 nm, where such values are very dependent on the nature of the endcapping group of the cumulene compound. It is worth stressing that from spectral data reported in [
20], the number of pi electrons in our polycumulene chain can be likely estimated in the range between 12–14, i.e., the chain should contain about 11–13 cumulated bonds. Furthermore, the presence of maximum absorption of such a high energy, i.e., 465 nm, reflects on the absence of conjugation with the endcapping group and the cumulene [
10].
In order to justify the UV-Vis optical results with those presented in
Table 1, the expected high nitrogen to carbon atomic ratio brings about that the amino group might only be an end-group, but also a bridge group, i.e., two cumulene fragments can be connected through the –NH– bridge. The role of the amino groups in the AC as chromophore groups should also be taken into account, as they would lower the energy of the electronic transition. Hence, the observed absorption spectrum agrees with shorter cumulene fragments. Moreover, the presence of branched structures with much lower energy and therefore a decrease in the Highest Occupied Molecular Orbital (HOMO)– Lowest Unoccupied Molecular Orbital (LUMO) energy gap should be likely considered.
Figure 3B shows the Raman spectrum of AC. Two intense overlapping peaks are observed at 1370 and 1570 cm
−1, which are characteristic of the low-ordered forms of carbon (usually denoted by D and G bands, respectively. The broad band featured at 2800 cm
−1 can be attributed to vibrations of N–H bonds. It is impossible to compare our Raman spectrum to data reported in the literature for various carbyne forms, i.e., polycumulenes and polyynes, because of the very large scattering of literature data, even for the same type of compounds. Hence, the strong influence of substituents on the position and especially the intensity of the bands in the Raman spectrum should be also taken into account.
The 4000–600 cm
−1 region of the IR spectrum of the AC compound showed absorption bands at 3156 s, 2926 s, 2857 s, 1631 s, 1461 s, 1378 m, 1092 s, 1036 s, and 762 w cm
−1. According to the literature [
4], cumulated double carbon–carbon bonds are featured by absorption bands in the 1600–1700 cm
−1 region. The band at 1631 cm
−1 is found within the aforementioned range for polycumulenes. In this case, it is broadened, since the substance is an oligomer, and the lateral C=C bonds are in a different environment rather than the C=C bonds in the main moiety, although such a band is superimposed on the vibrational band of the carbonyl group, due to the hydrolysis according to chemical reaction (10). A stretching vibrational mode near 2200 cm
−1, which is characteristic of the triple carbon–carbon bonds, is absent. A wide stretching vibrational mode at 3456 cm
−1 can be attributed to the vibrations of N–H bonds. The bands at 2926 cm
−1 and 2857 cm
−1 can be attributed to the stretching vibrations of C–H bonds, and the band at 1461 cm
−1 can be attributed to the bending vibrational mode of C–H bonds. For comparative purposes between the AC and HMTA IR spectra, the literature reported the 1300–200 cm
−1 region IR spectrum of the HMTA recorded in Nujol showing absorption bands at 1180 w, 1040 m, 1000 s, 823 w, 803 s, 714 w, 664 s, 610 m, and 508 s cm
−1 [
21]. Of these, the bands at 1225 and 1000 cm
−1 are assigned to the C–N stretching modes. In this regard, our bands at 1092 and 1036 cm
−1 can be likely attributed to the C–N stretching of the polycomulene compound.
3.3. Applications of AC as Dispersant Agent
The polycumulene AC might exhibit enhanced affinity for the surface of carbon materials, thereby acting as a dispersant and stabilizer of aqueous dispersions containing carbon nanotubes or graphene toward the preparation of nanoinks. In this respect,
Figure 4 shows the experimental and theoretical concentration of dispersing pristine and oxidized “Taunit-M” carbon nanotubes in aqueous acetic acid solution containing AC as dispersing agent. It is worth noticing that an analogous method for the spectrophotometric determination of carbon nanotubes concentration in solutions using various surfactants is described in [
22]. According to
Figure 4, it can be observed that the best results are achieved with oxidized CNTs compared with the pristine “Taunit-M” CNTs, as shown in
Figure 4A,B. Similar results were obtained earlier when the Taunit-M CNTs were dispersed using a water-soluble phenol-formaldehyde resin as a dispersant [
23]. As the amount of CNTs increases, the concentration of CNTs in the solution passes through a maximum, which is likely associated with the maximum adsorption of AC dispersant on the CNT surface. By contrast, if the CNT-to-AC ratio becomes too high, the surface concentration of the AC dispersant adsorbed on the CNTs becomes too small to keep CNTs dispersed in solution. However, an increment of the AC starting concentration leads to an increase in the maximum concentration of CNTs in solution, as depicted in
Figure 4C. By contrast, the use of traditional surfactants (for example, Triton X-100, sodium dodecylbenzenesulfonate, oil spill dispersant NF, polyvinylpyrrolidone) confirmed that the maximum achievable concentration of CNTs after ultrasonic dispersion in aqueous solution was two orders of magnitude smaller. With respect to the maximum achieved in CNTs concentration, AC as dispersant behaves similar to phenol-formaldehyde resin (PFR) [
23]. It is important to highlight several advantages of using AC instead of PFR. Firstly, PFR is used as a dispersant in slightly alkaline medium, while AC is used as a dispersant in acidic pH medium. Secondly, a significant difference is pointed out when drying CNT dispersions stabilized by PFR, making it impossible to obtain uniform films on surfaces, such as glass plates, because of CNTs’ aggregation. In addition, the dispersant aggregation during drying is practically discarded when using AC as a CNT, making the preparation of thin and uniform films onto different substrates feasible.
Similarly, another use of AC as a dispersant and stabilizer for carbon nanomaterials dispersions was also proven for the exfoliation of graphite. It is known that graphite and various graphitic materials, e.g., thermally expanded graphite or intercalated graphite, split into individual graphene nanoplatelets [
24] under the action of ultrasound in aqueous surfactant solutions. However, when using traditional surfactants, only a small fraction of the initial graphite concentration is split into thin nanoplatelets, providing a very low concentration in solution. As a result, the ultrasonic exfoliation of graphite is costly and inefficient. We compared the effectiveness of one of the traditional surfactants OP-7 (the Russian analogue for Triton X-100) and AC in the process of ultrasonic exfoliation of natural graphite GSM-2. The degree of exfoliation was estimated from the optical density of sample dispersions, which, as it was shown in the literature [
25], can be an indicator of the thickness of graphene nanoplatelets for comparative analysis. In this regard,
Figure 5 depicts the effective light absorption coefficient versus insonation time for graphite exfoliation in the presence of AC and OP-7 as dispersing agents, respectively, providing the former dispersant with the highest performance for dispersions of graphene nanoplatelets. Furthermore, under the same conditions using OP-7, the aggregation of graphene particles after ultrasound switching off occurs so quickly that it is impossible to take a sample for measurement; therefore, data points on the graph are unreproducible. The effectiveness of the use of AC as a dispersant and stabilizer of graphene dispersions nanoplatelets was also confirmed when using the expanded graphite compound (EGC) as a starting graphite material [
26].
Figure 6 shows the dependencies of the effective light absorption coefficient at 500 nm for graphene nanoplatelets produced by the ultrasonic exfoliation of EGC in aqueous solution containing Triton X-100 and AC in aqueous acetic acid solutions. The results show that the most effective exfoliation of EGC is achieved by using AC with a remarkable graphene concentration of 10 g/L). By contrast, the result of exfoliation using Triton X-100 was worse, even at lower graphene concentrations.
Thus, AC can find astonishing application in the technology of carbon nanomaterials for the production of nanoinks, thin-film coatings, nanocomposite materials, or in the production of graphene nanoplatelets. Another application of AC as a stabilizer for aqueous dispersions of nanoparticles is the preparation of colloidal solutions of polyaniline. We have found that polyaniline under ultrasonic treatment in aqueous AC containing acetic acid solution provides stable transparent colloidal solutions, thereby obtaining thin transparent films upon drying [Rus Patent RU 2641278].