Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices
Abstract
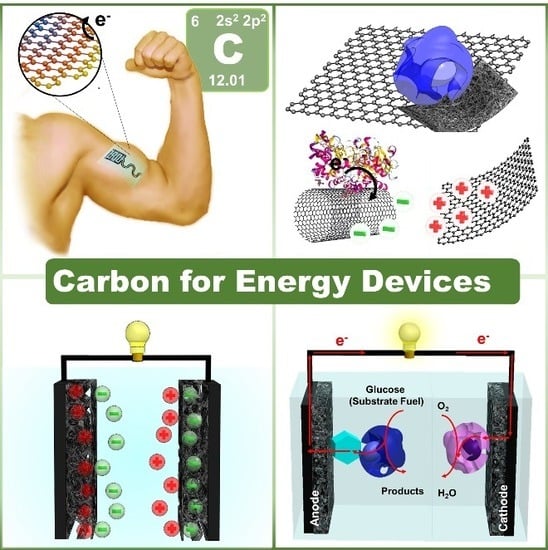
:1. Introduction
2. Moving Supercapacitors toward Personalized Platforms
3. Moving Biofuel Cells toward Personalized Platforms
4. Futuristic Self-Powered Biosensors Based on Biofuel Cells
5. Challenges in Carbon-Based Material Energy Devices and Potential Solutions
5.1. Electron Transfer between Carbon Materials and Enzymes
5.2. Oxygen Reduction Reaction in Biofuel Cells
5.3. Stability of Enzymes in Carbon-based Electrodes
5.4. Electrochemically Accessible Surface Area of Porous Carbon Materials
5.5. Mechanical Properties of Carbon-based Electrodes
6. Conclusions and Prospects
Conflicts of Interest
References
- Obreja, V.V.N. Supercapacitors Based on Carbon Nanomaterials. In Carbon Nanomaterials for Advanced Energy System; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 9, pp. 295–337. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.-B.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Cao, X.; Halder, A.; Tang, Y.; Hou, C.; Wang, H.; Duus, J.Ø.; Chi, Q. Engineering two-dimensional layered nanomaterials for wearable biomedical sensors and power devices. Mater. Chem. Front. 2018, 2, 1944–1986. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379. [Google Scholar] [CrossRef]
- Gao, B.; Wang, X.; Li, T.; Feng, Z.; Wang, C.; Gu, Z. Gecko-Inspired Paper Artificial Skin for Intimate Skin Contact and Multisensing. Adv. Mater. Technol. 2019, 4, 1800392. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Koydemir, H.C.; Ozcan, A. Wearable and Implantable Sensors for Biomedical Applications. Annu. Rev. Anal. Chem. 2018, 11, 127–146. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Wu, W. Recent progress in printed flexible solid-state supercapacitors for portable and wearable energy storage. J. Power Sources 2019, 410-411, 69–77. [Google Scholar] [CrossRef]
- Yun, J.; Song, C.; Lee, H.; Park, H.; Jeong, Y.R.; Kim, J.W.; Jin, S.W.; Oh, S.Y.; Sun, L.; Zi, G.; et al. Stretchable array of high-performance micro-supercapacitors charged with solar cells for wireless powering of an integrated strain sensor. Nano Energy 2018, 49, 644–654. [Google Scholar] [CrossRef]
- Falk, M.; Shleev, S. Hybrid dual-functioning electrodes for combined ambient energy harvesting and charge storage: Towards self-powered systems. Biosens. Bioelectron. 2019, 126, 275–291. [Google Scholar] [CrossRef]
- Jeerapan, I. Wearable Skin-Worn Enzyme-Based Electrochemical Devices: Biosensing, Energy Harvesting, and Self-Powered Sensing. In Wearable Devices; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Xiao, X.; Siepenkoetter, T.; Conghaile, P.Ó.; Leech, D.; Magner, E. Nanoporous Gold-Based Biofuel Cells on Contact Lenses. ACS Appl. Mater. Interfaces 2018, 10, 7107–7116. [Google Scholar] [CrossRef]
- Valdés-Ramírez, G.; Li, Y.-C.; Kim, J.; Jia, W.; Bandodkar, A.J.; Nuñez-Flores, R.; Miller, P.R.; Wu, S.-Y.; Narayan, R.; Windmiller, J.R.; et al. Microneedle-based self-powered glucose sensor. Electrochem. Commun. 2014, 47, 58–62. [Google Scholar] [CrossRef]
- Güven, G.; Şahin, S.; Güven, A.; Yu, E.H. Power Harvesting from Human Serum in Buckypaper-Based Enzymatic Biofuel Cell. Front. Energy Res. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Li, Y.; Feng, H.; Lu, J.; Li, J. Preparation, Structure, and Electrochemical Properties of Reduced Graphene Sheet Films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Tanaka, K.; Iijima, S. Carbon Nanotubes and Graphene; Elsevier (Newnes): Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sun, Y.-P.; Fu, K.; Lin, Y.; Huang, W. Functionalized Carbon Nanotubes: Properties and Applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Lemine, A.S.; Zagho, M.M.; Altahtamouni, T.M.; Bensalah, N. Graphene a promising electrode material for supercapacitors—A review. Int. J. Energy Res. 2018, 42, 4284–4300. [Google Scholar] [CrossRef]
- Jewell, D.; Duong, H.; Cheng, H. Advanced Supercapacitors Using Carbon Nanotubes. Mater. Res. Found. 2017, 12, 207–228. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: a review. J. Mater. Chem. C 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2014, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Rajendran, V.; Mohan, A.M.V.; Jayaraman, M.; Nakagawa, T. All-printed, interdigitated, freestanding serpentine interconnects based flexible solid state supercapacitor for self powered wearable electronics. Nano Energy 2019, 65, 104055. [Google Scholar] [CrossRef]
- Daneshvar, F.; Aziz, A.; Abdelkader, A.M.; Zhang, T.; Sue, H.-J.; Welland, M.E. Porous SnO2–Cu x O nanocomposite thin film on carbon nanotubes as electrodes for high performance supercapacitors. Nanotechnology 2018, 30, 015401. [Google Scholar] [CrossRef]
- Lv, J.; Jeerapan, I.; Tehrani, F.; Yin, L.; Silva-Lopez, C.A.; Jang, J.-H.; Joshuia, D.; Shah, R.; Liang, Y.; Xie, L.; et al. Sweat-based wearable energy harvesting-storage hybrid textile devices. Energy Environ. Sci. 2018, 11, 3431–3442. [Google Scholar] [CrossRef]
- Feng, N.; Meng, R.; Zu, L.; Feng, Y.; Peng, C.; Huang, J.; Liu, G.; Chen, B.; Yang, J. A polymer-direct-intercalation strategy for MoS2/carbon-derived heteroaerogels with ultrahigh pseudocapacitance. Nat. Commun. 2019, 10, 1372. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, H.; Yang, W.; Liu, Y.; Cao, X.; Zhang, J.; Li, C.; Liu, J.; Gooding, J.J. Screen-printable films of graphene/CoS2/Ni3S4 composites for the fabrication of flexible and arbitrary-shaped all-solid-state hybrid supercapacitors. Carbon 2019, 146, 557–567. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Gao, S.; Wang, K.; Du, Z.; Wang, Y.; Yuan, A.; Lu, W.; Chen, L. High power density electric double-layer capacitor based on a porous multi-walled carbon nanotube microsphere as a local electrolyte micro-reservoir. Carbon 2015, 92, 254–261. [Google Scholar] [CrossRef]
- Zhou, G.; Kim, N.-R.; Chun, S.-E.; Lee, W.; Um, M.-K.; Chou, T.-W.; Islam, M.F.; Byun, J.-H.; Oh, Y. Highly porous and easy shapeable poly-dopamine derived graphene-coated single walled carbon nanotube aerogels for stretchable wire-type supercapacitors. Carbon 2018, 130, 137–144. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. An overview of graphene in energy production and storage applications. J. Power Source 2011, 196, 4873–4885. [Google Scholar] [CrossRef]
- Xiong, G.; Meng, C.; Reifenberger, R.G.; Irazoqui, P.P.; Fisher, T.S. A Review of Graphene-Based Electrochemical Microsupercapacitors. Electroanalysis 2014, 26, 30–51. [Google Scholar] [CrossRef]
- Stoner, B.R.; Raut, A.S.; Brown, B.; Parker, C.B.; Glass, J.T. Graphenated carbon nanotubes for enhanced electrochemical double layer capacitor performance. Appl. Phys. Lett. 2011, 99, 183104. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Zhuang, X.; Xu, Z.; Xie, Y.; Zhu, X.; Xu, Y.; Sun, B.; Lin, J.; Zhang, Y.; Yan, Z. Mechanically Assembled, Three-Dimensional Hierarchical Structures of Cellular Graphene with Programmed Geometries and Outstanding Electromechanical Properties. ACS Nano 2018, 12, 12456–12463. [Google Scholar] [CrossRef]
- Manjakkal, L.; Núñez, C.G.; Dang, W.; Dahiya, R. Flexible self-charging supercapacitor based on graphene-Ag-3D graphene foam electrodes. Nano Energy 2018, 51, 604–612. [Google Scholar] [CrossRef]
- Xu, T.; Yang, D.; Fan, Z.; Li, X.; Liu, Y.; Guo, C.; Zhang, M.; Yu, Z.-Z. Reduced graphene oxide/carbon nanotube hybrid fibers with narrowly distributed mesopores for flexible supercapacitors with high volumetric capacitances and satisfactory durability. Carbon 2019, 152, 134–143. [Google Scholar] [CrossRef]
- Han, J.; Wang, H.; Yue, Y.; Mei, C.; Chen, J.; Huang, C.; Wu, Q.; Xu, X. A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon 2019, 149, 1–18. [Google Scholar] [CrossRef]
- Sim, H.J.; Choi, C.; Lee, D.Y.; Kim, H.; Yun, J.-H.; Kim, J.M.; Kang, T.M.; Ovalle, R.; Baughman, R.H.; Kee, C.W.; et al. Biomolecule based fiber supercapacitor for implantable device. Nano Energy 2018, 47, 385–392. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.-q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef] [PubMed]
- Jeerapan, I.; Sempionatto, J.R.; Wang, J. On-Body Bioelectronics: Wearable Biofuel Cells for Bioenergy Harvesting and Self-Powered Biosensing. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Grattieri, M.; Minteer, S.D. Self-Powered Biosensors. ACS Sens. 2018, 3, 44–53. [Google Scholar] [CrossRef]
- Fu, L.; Liu, J.; Hu, Z.; Zhou, M. Recent Advances in the Construction of Biofuel Cells Based Self-powered Electrochemical Biosensors: A Review. Electroanalysis 2018, 30, 2535–2550. [Google Scholar] [CrossRef]
- Katz, E. Boolean Logic Gates Realized with Enzyme-catalyzed Reactions – Unusual Look at Usual Chemical Reactions. ChemPhysChem 2019, 20, 9–22. [Google Scholar] [CrossRef]
- El Ichi-Ribault, S.; Alcaraz, J.-P.; Boucher, F.; Boutaud, B.; Dalmolin, R.; Boutonnat, J.; Cinquin, P.; Zebda, A.; Martin, D.K. Remote wireless control of an enzymatic biofuel cell implanted in a rabbit for 2 months. Electrochim. Acta 2018, 269, 360–366. [Google Scholar] [CrossRef]
- Prasad, K.P.; Chen, Y.; Chen, P. Three-Dimensional Graphene-Carbon Nanotube Hybrid for High-Performance Enzymatic Biofuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 3387–3393. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, M.; Yang, L.; Tan, Y.; Deng, W.; Ma, M.; Su, X.; Xie, Q. Three-Dimensional Graphene Networks as a New Substrate for Immobilization of Laccase and Dopamine and Its Application in Glucose/O2 Biofuel Cell. ACS Appl. Mater. Interfaces 2014, 6, 12808–12814. [Google Scholar] [CrossRef]
- Mazurenko, I.; Monsalve, K.; Rouhana, J.; Parent, P.; Laffon, C.; Goff, A.L.; Szunerits, S.; Boukherroub, R.; Giudici-Orticoni, M.-T.; Mano, N.; et al. How the Intricate Interactions between Carbon Nanotubes and Two Bilirubin Oxidases Control Direct and Mediated O2 Reduction. ACS Appl. Mater. Interfaces 2016, 8, 23074–23085. [Google Scholar] [CrossRef]
- Campbell, A.S.; Jeong, Y.J.; Geier, S.M.; Koepsel, R.R.; Russell, A.J.; Islam, M.F. Membrane/Mediator-Free Rechargeable Enzymatic Biofuel Cell Utilizing Graphene/Single-Wall Carbon Nanotube Cogel Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 4056–4065. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; You, J.-M.; Nuñez-Flores, R.; Wang, J. Highly Stretchable Fully-Printed CNT-Based Electrochemical Sensors and Biofuel Cells: Combining Intrinsic and Design-Induced Stretchability. Nano Lett. 2016, 16, 721–727. [Google Scholar] [CrossRef]
- Sim, H.J.; Lee, D.Y.; Kim, H.; Choi, Y.-B.; Kim, H.-H.; Baughman, R.H.; Kim, S.J. Stretchable Fiber Biofuel Cell by Rewrapping Multiwalled Carbon Nanotube Sheets. Nano Lett. 2018, 18, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, A.; Murata, K.; Shigemori, Y.; Zebda, A.; Tsujimura, S. High-performance enzymatic biofuel cell based on flexible carbon cloth modified with MgO-templated porous carbon. J. Power Source 2019, 427, 49–55. [Google Scholar] [CrossRef]
- Agnès, C.; Holzinger, M.; Le Goff, A.; Reuillard, B.; Elouarzaki, K.; Tingry, S.; Cosnier, S. Supercapacitor/biofuel cell hybrids based on wired enzymes on carbon nanotube matrices: autonomous reloading after high power pulses in neutral buffered glucose solutions. Energy Environ. Sci. 2014, 7, 1884–1888. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Chen, T. Chapter 15—Carbon Nanotube and Graphene Fibers for Wearable Fiber-Shaped Energy Conversion. In Nanotube Superfiber Materials (Second Edition); Schulz, M.J., Shanov, V., Yin, Z., Cahay, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 359–381. [Google Scholar] [CrossRef]
- Tsujimura, S.; Murata, K. Electrochemical Oxygen Reduction Catalyzed by Bilirubin Oxidase with the Aid of 2,2′-Azinobis(3-ethylbenzothiazolin-6-sulfonate) on a MgO-template Carbon Electrode. Electrochim. Acta 2015, 180, 555–559. [Google Scholar] [CrossRef]
- Morishita, T.; Tsumura, T.; Toyoda, M.; Przepiórski, J.; Morawski, A.W.; Konno, H.; Inagaki, M. A review of the control of pore structure in MgO-templated nanoporous carbons. Carbon 2010, 48, 2690–2707. [Google Scholar] [CrossRef]
- Tsujimura, S.; Oyama, M.; Funabashi, H.; Ishii, S. Effects of pore size and surface properties of MgO-templated carbon on the performance of bilirubin oxidase–modified oxygen reduction reaction cathode. Electrochim. Acta 2019, 322, 134744. [Google Scholar] [CrossRef]
- Yeknami, A.F.; Wang, X.; Jeerapan, I.; Imani, S.; Nikoofard, A.; Wang, J.; Mercier, P.P. A 0.3-V CMOS Biofuel-Cell-Powered Wireless Glucose/Lactate Biosensing System. IEEE J. Solid-State Circuits 2018, 53, 3126–3139. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2019, 1902670. [Google Scholar] [CrossRef]
- Slaughter, G.; Kulkarni, T. Highly Selective and Sensitive Self-Powered Glucose Sensor Based on Capacitor Circuit. Sci. Rep. 2017, 7, 1471. [Google Scholar] [CrossRef]
- Yuen, J.D.; Baingane, A.; Hasan, Q.; Shriver-Lake, L.C.; Walper, S.A.; Zabetakis, D.; Breger, J.C.; Stenger, D.A.; Slaughter, G. A Fully-Flexible Solution-Processed Autonomous Glucose Indicator. Sci. Rep. 2019, 9, 6931. [Google Scholar] [CrossRef]
- Shitanda, I.; Fujimura, Y.; Nohara, S.; Hoshi, Y.; Itagaki, M.; Tsujimura, S. Paper-Based Disk-Type Self-Powered Glucose Biosensor Based on Screen-Printed Biofuel Cell Array. J. Electrochem. Soc. 2019, 166, B1063–B1068. [Google Scholar] [CrossRef]
- Gross, A.J.; Holzinger, M.; Cosnier, S. Buckypaper bioelectrodes: emerging materials for implantable and wearable biofuel cells. Energy Environ. Sci. 2018, 11, 1670–1687. [Google Scholar] [CrossRef]
- Chen, X.; Yin, L.; Lv, J.; Gross, A.J.; Le, M.; Gutierrez, N.G.; Li, Y.; Jeerapan, I.; Giroud, F.; Berezovska, A.; et al. Stretchable and Flexible Buckypaper-Based Lactate Biofuel Cell for Wearable Electronics. Adv. Funct. Mater. 2019, 1905785. [Google Scholar] [CrossRef]
- Heller, A. Electrical wiring of redox enzymes. Acc. Chem. Res. 1990, 23, 128–134. [Google Scholar] [CrossRef]
- Conzuelo, F.; Marković, N.; Ruff, A.; Schuhmann, W. The Open Circuit Voltage in Biofuel Cells: Nernstian Shift in Pseudocapacitive Electrodes. Angew. Chem. Int. Ed. 2018, 57, 13681–13685. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Glennon, J.D.; Gedanken, A.; Vashist, S.K. Achievement and assessment of direct electron transfer of glucose oxidase in electrochemical biosensing using carbon nanotubes, graphene, and their nanocomposites. Microchimica Acta 2017, 184, 369–388. [Google Scholar] [CrossRef]
- Wooten, M.; Karra, S.; Zhang, M.; Gorski, W. On the Direct Electron Transfer, Sensing, and Enzyme Activity in the Glucose Oxidase/Carbon Nanotubes System. Anal. Chem. 2014, 86, 752–757. [Google Scholar] [CrossRef]
- Willner, I.; Yan, Y.-M.; Willner, B.; Tel-Vered, R. Integrated Enzyme-Based Biofuel Cells–A Review. Fuel Cells 2009, 9, 7–24. [Google Scholar] [CrossRef]
- Yoshino, S.; Miyake, T.; Yamada, T.; Hata, K.; Nishizawa, M. Molecularly Ordered Bioelectrocatalytic Composite Inside a Film of Aligned Carbon Nanotubes. Adv. Energy Mater. 2013, 3, 60–64. [Google Scholar] [CrossRef]
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic fuel cells: Recent progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Reuillard, B.; Le Goff, A.; Agnes, C.; Holzinger, M.; Zebda, A.; Gondran, C.; Elouarzaki, K.; Cosnier, S. High power enzymatic biofuel cell based on naphthoquinone-mediated oxidation of glucose by glucose oxidase in a carbon nanotube 3D matrix. Phys. Chem. Chem. Phys. 2013, 15, 4892–4896. [Google Scholar] [CrossRef]
- Jeerapan, I.; Ciui, B.; Martin, I.; Cristea, C.; Sandulescu, R.; Wang, J. Fully edible biofuel cells. J. Mater. Chem. B 2018, 6, 3571–3578. [Google Scholar] [CrossRef]
- De Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New trends in enzyme immobilization at nanostructured interfaces for efficient electrocatalysis in biofuel cells. Electrochim. Acta 2014, 126, 104–114. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Carbon nanotube/enzyme biofuel cells. Electrochim. Acta 2012, 82, 179–190. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Al-Lolage, F.A. There is no evidence to support literature claims of direct electron transfer (DET) for native glucose oxidase (GOx) at carbon nanotubes or graphene. J. Electroanal. Chem. 2018, 819, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Baek, S.; Lee, H.; Reginald, S.S.; Kim, Y.; Kang, H.; Choi, I.-G.; Chang, I.S. Construction of Uniform Monolayer- and Orientation-Tunable Enzyme Electrode by a Synthetic Glucose Dehydrogenase without Electron-Transfer Subunit via Optimized Site-Specific Gold-Binding Peptide Capable of Direct Electron Transfer. ACS Appl. Mater. Interfaces 2018, 10, 28615–28626. [Google Scholar] [CrossRef]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Barton, S.C.; Kim, H.-H.; Binyamin, G.; Zhang, Y.; Heller, A. The “Wired” Laccase Cathode: High Current Density Electroreduction of O2 to Water at +0.7 V (NHE) at pH 5. J. Am. Chem. Soc. 2001, 123, 5802–5803. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Jian, G.; Lei, J.; Hu, Z.; Ju, H. A glucose biosensor based on direct electrochemistry of glucose oxidase immobilized on nitrogen-doped carbon nanotubes. Biosens. Bioelectron. 2009, 25, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760. [Google Scholar] [CrossRef]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. 1-Oxygen Solubility, Diffusion Coefficient, and Solution Viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, M.; Bai, L.; Han, L.; Dong, S. Recoverable hybrid enzymatic biofuel cell with molecular oxygen-independence. Biosens. Bioelectron. 2016, 75, 23–27. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; You, J.-M.; Wang, J. Enzymatic glucose/oxygen biofuel cells: Use of oxygen-rich cathodes for operation under severe oxygen-deficit conditions. Biosens. Bioelectron. 2018, 122, 284–289. [Google Scholar] [CrossRef]
- Kontani, R.; Tsujimura, S.; Kano, K. Air diffusion biocathode with CueO as electrocatalyst adsorbed on carbon particle-modified electrodes. Bioelectrochemistry 2009, 76, 10–13. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Cepra, G. Thermal Stabilization of Enzymes Immobilized within Carbon Paste Electrodes. Anal. Chem. 1997, 69, 3124–3127. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M.; Mo, J.-W. Acid Stability of Carbon Paste Enzyme Electrodes. Anal. Chem. 2006, 78, 7044–7047. [Google Scholar] [CrossRef]
- Sotiropoulou, S.; Vamvakaki, V.; Chaniotakis, N.A. Stabilization of enzymes in nanoporous materials for biosensor applications. Biosens. Bioelectron. 2005, 20, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845. [Google Scholar] [CrossRef] [PubMed]
- Pierotti, R.; Rouquerol, J. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem 1985, 57, 603–619. [Google Scholar]
- Mazurenko, I.; de Poulpiquet, A.; Lojou, E. Recent developments in high surface area bioelectrodes for enzymatic fuel cells. Curr. Opin. Electrochem. 2017, 5, 74–84. [Google Scholar] [CrossRef]
- Flexer, V.; Brun, N.; Backov, R.; Mano, N. Designing highly efficient enzyme-based carbonaceous foams electrodes for biofuel cells. Energy Environ. Sci. 2010, 3, 1302–1306. [Google Scholar] [CrossRef]
- Funabashi, H.; Takeuchi, S.; Tsujimura, S. Hierarchical meso/macro-porous carbon fabricated from dual MgO templates for direct electron transfer enzymatic electrodes. Sci. Rep. 2017, 7, 45147. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Kim, J.; Wang, Y.; Huang, H.; Kim, Y. Flexible and wearable fiber shaped high voltage supercapacitors based on copper hexacyanoferrate and porous carbon coated carbon fiber electrodes. J. Mater. Chem. C 2016, 4, 4934–4940. [Google Scholar] [CrossRef]
- Yu, J.; Lu, W.; Pei, S.; Gong, K.; Wang, L.; Meng, L.; Huang, Y.; Smith, J.P.; Booksh, K.S.; Li, Q.; et al. Omnidirectionally Stretchable High-Performance Supercapacitor Based on Isotropic Buckled Carbon Nanotube Films. ACS Nano 2016, 10, 5204–5211. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.-H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Nam, I.; Bae, S.; Park, S.; Yoo, Y.G.; Lee, J.M.; Han, J.W.; Yi, J. Omnidirectionally stretchable, high performance supercapacitors based on a graphene–carbon-nanotube layered structure. Nano Energy 2015, 15, 33–42. [Google Scholar] [CrossRef]
- Laoui, T. Mechanical and Thermal Properties of Styrene Butadiene Rubber - Functionalized Carbon Nanotubes Nanocomposites. Fuller. Nanotubes Carbon Nanostruct. 2013, 21, 89–101. [Google Scholar] [CrossRef]
- Kyrylyuk, A.V.; Hermant, M.C.; Schilling, T.; Klumperman, B.; Koning, C.E.; van der Schoot, P. Controlling electrical percolation in multicomponent carbon nanotube dispersions. Nat. Nanotechnol. 2011, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Mitra, D.; Peterson, K.; Maharbiz, M.M. A Highly Elastic, Capacitive Strain Gauge Based on Percolating Nanotube Networks. Nano Lett. 2012, 12, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Wang, X.; Zhu, Y.; Zhu, Y. Wavy Ribbons of Carbon Nanotubes for Stretchable Conductors. Adv. Funct. Mater. 2012, 22, 1279–1283. [Google Scholar] [CrossRef]
- Wisniewski, N.; Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces 2000, 18, 197–219. [Google Scholar] [CrossRef]
- Barfidokht, A.; Gooding, J.J. Approaches Toward Allowing Electroanalytical Devices to be Used in Biological Fluids. Electroanalysis 2014, 26, 1182–1196. [Google Scholar] [CrossRef]
- Wang, B.; Yang, P.; Ding, Y.; Qi, H.; Gao, Q.; Zhang, C. Improvement of the Biocompatibility and Potential Stability of Chronically Implanted Electrodes Incorporating Coating Cell Membranes. ACS Appl. Mater. Interfaces 2019, 11, 8807–8817. [Google Scholar] [CrossRef]
- Cadet, M.; Gounel, S.; Stines-Chaumeil, C.; Brilland, X.; Rouhana, J.; Louerat, F.; Mano, N. An enzymatic glucose/O2 biofuel cell operating in human blood. Biosens. Bioelectron. 2016, 83, 60–67. [Google Scholar] [CrossRef]
- Escalona-Villalpando, R.A.; Ortiz-Ortega, E.; Bocanegra-Ugalde, J.P.; Minteer, S.D.; Ledesma-García, J.; Arriaga, L.G. Clean energy from human sweat using an enzymatic patch. J. Power Sources 2019, 412, 496–504. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeerapan, I.; Ma, N. Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices. C 2019, 5, 62. https://doi.org/10.3390/c5040062
Jeerapan I, Ma N. Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices. C. 2019; 5(4):62. https://doi.org/10.3390/c5040062
Chicago/Turabian StyleJeerapan, Itthipon, and Nicolás Ma. 2019. "Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices" C 5, no. 4: 62. https://doi.org/10.3390/c5040062
APA StyleJeerapan, I., & Ma, N. (2019). Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices. C, 5(4), 62. https://doi.org/10.3390/c5040062