A Novel Mesoporous Carbon as Potential Conductive Additive for a Li-Ion Battery Cathode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Mesoporous Carbon (MC)
2.3. Preparation of Composites LiFePO4/MC and LiFEPO4/Super P
2.4. Physical and Chemical Characterization of Carbons and Composites
2.5. Electrochemical Characterization of the Composites LiFePO4/MC and LiFePO4/Super P
3. Results and Discussion
3.1. Porous and Morphological Characteristics of Carbon Samples
Origin of the Bimodal Porosity of MC
3.2. Structural Characterization
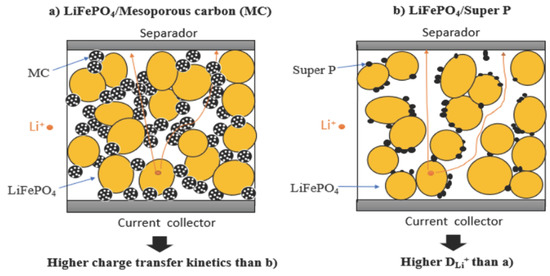
3.3. Physical and Chemical Characterization of LiFePO4/MC and LiFePO4/Super P
3.4. Electrochemical Performance of Lithium-Ion Battery
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Wang, H.; Liu, H.; Liu, J.; Yan, H. Research Progress in Improving the Rate Performance of LiFePO4 Cathode Materials. Nano-Micro Lett. 2014, 6, 209–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, Q.Y.; Du, P.P.; Wang, L.Z.; Zhang, A.Q.; Song, Y.H.; Lv, Y.; Li, G.Y. Advances in new cathode material LiFePO4 for lithium-ion batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. Electrochem. Sci. Tech. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Yuan, L.-X.; Wu, M.; Sun, D.; Huang, Y.-H. Effects of Na+ and Cl- co-doping on electrochemical performance in LiFePO4/C. Electrochim. Acta 2011, 56, 8477–8483. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, J.; Guo, Z.; Tong, Q.; Huang, W.; Li, B. Synthesis of LiFe1−xNixPO4/C composites and their electrochemical performance. J. Power Sources 2009, 194, 786–793. [Google Scholar] [CrossRef]
- Saravanan, K.; Balaya, P.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Morphology controlled synthesis of LiFePO4/C nanoplates for Li-ion batteries. Energy Environ. Sci. 2010, 3, 457–463. [Google Scholar] [CrossRef]
- Delacourt, C.; Poizot, P.; Levasseur, S.; Masquelier, C. Size Effects on Carbon-Free LiFePO4 Powders. The key to superior energy density. Solid-State Lett. 2006, 9, A352–A355. [Google Scholar] [CrossRef]
- Park, K.S.; Son, J.T.; Chung, H.T.; Kim, S.J.; Lee, C.H.; Kang, K.T.; Kim, H.G. Surface modification by silver coating for improving electrochemical properties of LiFePO4. Solid State Commun. 2004, 129, 311–314. [Google Scholar] [CrossRef]
- Dominko, R.; Bele, M.; Goupil, J.M.; Gaberscek, M.; Hanzel, D.; Arcon, I.; Jamnik, J. Wired porous cathode materials: A novel concept for synthesis of LiFePO4. Chem. Mater. 2007, 19, 2960–2969. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, Y.R.; Hosono, E.; Wang, K.X.; Zhou, H.S. The design of a LiFePO4/carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method. Angew. Chem. Int. Ed. 2008, 47, 7461–7465. [Google Scholar] [CrossRef]
- Huang, H.; Yin, S.C.; Nazar, L.F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates. Electrochem. Solid State Lett. 2001, 4, A170–A172. [Google Scholar] [CrossRef]
- Croce, F.; Epifanio, A.D.; Hassoun, J.; Deptula, A.; Olczac, T.; Scrosati, B. A novel concept for the synthesis of an improved LiFePO4 lithium battery cathode. Electrochem. Solid State Lett. 2002, 5, A47–A50. [Google Scholar] [CrossRef] [Green Version]
- Doherty, C.M.; Caruso, R.A.; Smarsly, B.M.; Adelhelm, P.; Drummond, C.J. Hierarchically porous monolithic LiFePO4/carbon composite electrode materials for high power lithium ion batteries. Chem. Mater. 2009, 21, 5300–5306. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.-J.; Yang, Y.-F.; Song, G.-Z. Up-scalable synthesis, structure and charge storage properties of porous microspheres of LiFePO4@C nanocomposites. Mater. Chem. 2009, 19, 9121–9125. [Google Scholar] [CrossRef]
- Oh, S.W.; Myung, S.-T.; Bang, H.J.; Yoon, C.S.; Amine, K.; Sun, Y.K. Nanoporous structured LiFePO4 with spherical microscale particles having high volumetric capacity for lithium batteries. Electrochem. Solid-State Lett. 2009, 12, A181–A185. [Google Scholar] [CrossRef]
- Chung, S.Y.; Bloking, J.T.; Chiang, Y.M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef]
- Wang, G.X.; Bewlay, S.; Yao, J.; Ahn, J.H.; Dou, S.X.; Liu, H.K. Characterization of LiMxFe1 − xPO4 (M = Mg, Zr, Ti) cathode materials prepared by the sol-gel method. Electrochem. Solid State Lett. 2004, 7, A503–A506. [Google Scholar] [CrossRef]
- Sarkar, S.; Mitra, S. Carbon coated submicron sized-LiFePO4: Improved high rate performance lithium battery cathode. Energy Procedia 2014, 54, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Franger, S.; Le Cras, F.; Bourbon, C.; Rouault, H. LiFePO4 synthesis routes for enhanced electrochemical performance. Electrochem. Solid State Lett. 2002, 5, A231–A233. [Google Scholar] [CrossRef]
- Liu, J.; Conry, T.E.; Song, X.; Doeff, M.M.; Richardson, T.J. Nanoporous spherical LiFePO4 for high performance cathodes. Energy Environ. Sci. 2011, 4, 885–888. [Google Scholar] [CrossRef]
- Konarova, M.; Taniguchi, I. Synthesis of carbon-coated LiFePO4 nanoparticles with high rate performance in lithium secondary batteries. J. Power Sour. 2010, 195, 3661–3667. [Google Scholar] [CrossRef]
- Hasegawa, G.; Ishihara, Y.; Kanamori, K.; Miyazaki, K.; Yamada, Y.; Nakanishi, K.; Abe, T. Facile preparation of monolithic LiFePO4/carbon composites with well-defined macropores for a lithium-ion battery. Chem. Mater. 2011, 23, 5208–5216. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Fan, L.-Z. Carbon-coated LiFePO4–porous carbon composites as cathode materials for lithium ion batteries. Nanoscale 2013, 5, 2164–2168. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H. Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv. Mater. 2010, 22, 4944–4948. [Google Scholar] [CrossRef]
- Yu, L.; Cai, D.; Wang, H.; Titirici, M.-M. Synthesis of microspherical LiFePO4-carbon composites for lithium-ion batteries. Nanomaterials 2013, 3, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Li, D.; Lu, A.; Li, W. Controllable synthesis of high loading LiFePO4/C nanocomposites using bimodal mesoporous carbon as support for high power Li-ion battery cathodes. J. Ener. Chem. 2013, 22, 907–913. [Google Scholar] [CrossRef]
- Sun, S.; Ghimbeu, C.M.; Janot, R.; Le Meins, J.-M.; Cassel, A.; Davoisne, C.; Masquelier, C.; Vix-Guterl, C. One-pot synthesis of LiFePO4–carbon mesoporous composites for Li-ion batteries. Microp. Mesop. Mater. 2014, 198, 175–184. [Google Scholar] [CrossRef]
- Santa, C.F.; Jaber, M.; Guth, J.L.; Sierra, L. Synthesis of texturally biphasic mesoporous carbon-silica composites and carbons. Microp. Mesop. Mater. 2013, 173, 53–63. [Google Scholar] [CrossRef]
- Guzmán, G.; Vazquez-Arenas, J.; Ramos-Sánchez, G.; Bautista-Ramírez, M.; González, I. Improved performance of LiFePO4 cathode for Li-ion batteries through percolation studies. Electrochem. Acta 2017, 247, 451–459. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Martín-Martinez, J.M.; Prado-Burguete, C.; McEnaney, B. A standard adsorption isotherm for the characterization of activated carbons. J. Phys. Chem. 1987, 91, 515–516. [Google Scholar] [CrossRef]
- Manoj, B.; Kunjomana, A.G. Structural characterization of graphene layers in various Indian coals by X-ray Diffraction. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012096–012100. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Takai, K.; Enoki, T. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106–163108. [Google Scholar] [CrossRef]
- Sakintuna, B.; Yürüm, Y.; Çetinkaya, S. Evolution of carbon microstructures during the pyrolysis of Turkish Elbistan lignite in the temperature range 700−1000 °C. Energy Fuels 2004, 18, 883–888. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Li, W.; Guo, X.; Wang, Y.; Wang, G.; Zhao, D. Porous carbon composites for next generation rechargeable lithium batteries. Adv. Energy Mater. 2017, 7, 1614–1683. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Chrobak, T.; Ender, M.; Illig, J.; Klotz, D.; Ivers-Tiffée, E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J. Power Sour. 2011, 196, 5342–5348. [Google Scholar] [CrossRef]
- Illig, J.; Ender, M.; Chrobak, T.; Schmidt, J.P.; Klotz, D.; Ivers-Tiffée, E. Separation of charge transfer and contact resistance in LiFePO4-cathodes by impedance modeling. J. Electrochem. Soc. 2012, 159, A952–A960. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chen, J.S. A study of electrochemical performance of LiFePO4/C composites doped with Na and V. Int. J. Electrochem. Sci. 2012, 7, 8128–8139. [Google Scholar]









| Carbon | Vp (cm3/g) | SBET (m2/g) | Dpore (nm) | Amicro (m2/g) | Vmicro (cm3/g) |
|---|---|---|---|---|---|
| MC | 1.82 | 1198 | 3.9; 8.2 | 75 | 0.03 |
| Super P | 0.21 | 57 | 2.1; 59 | - | 0.08 |
| Carbon | ID/IG | La (nm) | Lc (nm) | d002 | n | TD (°C) |
|---|---|---|---|---|---|---|
| MC | 1.40 | 13.3 | 1.36 | 0.38 | 3.56 | 550–680 |
| Super P | 0.84 | 22.9 | 8.12 | 0.35 | 23.20 | 650–750 |
| Sample | Vp (cm3/g) | SBET (m2/g) | Dpore (nm) | Vmicro (cm3/g) | V2–10 nm (cm3/g) |
|---|---|---|---|---|---|
| LiFePO4/MC | 0.16 | 84 | 3.6, 7.7 | 0.0007 | 0.12 |
| LiFePO4/Super P | 0.06 | 14 | - | 0.0001 | 0 |
| Cathode Material | Before Cycling, DLi+ (cm2s−1) | After 11 Cycles, DLi+ (cm2s−1) |
|---|---|---|
| LiFePO4/MC | 1.7 × 10−10 | 1.3 × 10−14 |
| LiFePO4/Super P | 5.3 × 10−10 | 8.3 × 10−12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez, V.; López, B.; Palacio, R.; Sierra, L. A Novel Mesoporous Carbon as Potential Conductive Additive for a Li-Ion Battery Cathode. C 2019, 5, 81. https://doi.org/10.3390/c5040081
Vélez V, López B, Palacio R, Sierra L. A Novel Mesoporous Carbon as Potential Conductive Additive for a Li-Ion Battery Cathode. C. 2019; 5(4):81. https://doi.org/10.3390/c5040081
Chicago/Turabian StyleVélez, Victor, Betty López, Ruben Palacio, and Ligia Sierra. 2019. "A Novel Mesoporous Carbon as Potential Conductive Additive for a Li-Ion Battery Cathode" C 5, no. 4: 81. https://doi.org/10.3390/c5040081
APA StyleVélez, V., López, B., Palacio, R., & Sierra, L. (2019). A Novel Mesoporous Carbon as Potential Conductive Additive for a Li-Ion Battery Cathode. C, 5(4), 81. https://doi.org/10.3390/c5040081