Removal of F− from Water Using Templated Mesoporous Carbon Modified with Hydrated Zirconium Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mesoporous Carbons Modified with ZrO2·xH2O
2.3. Characterization of the Prepared Adsorbents
2.4. F− Adsorption Experiments
3. Results and Discussion
3.1. Characterization of Prepared Adsorbents
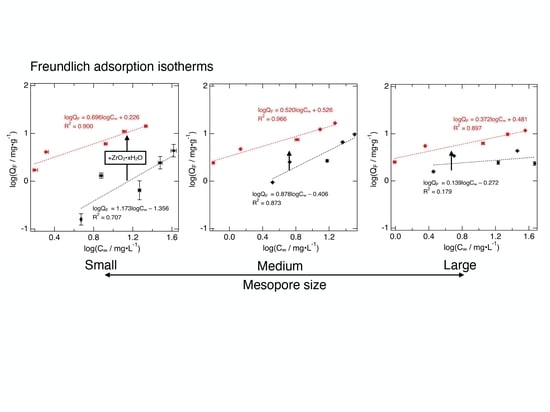
3.2. Freundlich Isotherms for F− Adsorption
3.3. Effect of pH on F− Adsorption
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, S. Review of fluoride removal from water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Vences-Alvarez, E.; Velazquez-Jimenez, L.H.; Chazaro-Ruiz, L.F.; Diaz-Flores, P.E.; Rangel-Mendez, J.R. Fluoride removal in water by a hybrid adsorbent lanthanum-carbon. J. Colloid Interfaces Sci. 2015, 455, 194–202. [Google Scholar] [CrossRef]
- Chen, L.; He, S.; Wang, T.-J.; Su, C.-L.; Zhang, C.; Jin, Y. Synthesis of Iron-Doped Titanium oxide Nanoadsorbent and Its Adsorption Characteristics for Fluoride in Drinking Water. Ind. Eng. Chem. Res. 2012, 51, 13150–13156. [Google Scholar] [CrossRef]
- Chen, L.; He, B.-Y.; He, S.; Wang, T.-J.; Su, C.-L.; Jin, Y. Fe-Ti oxide nano-adsorbent synthesized by co-precipitation for fluoride removal from drinking water and its adsorption mechanism. Powder Technol. 2012, 227, 3–8. [Google Scholar] [CrossRef]
- Cai, H.-M.; Chen, G.-J.; Peng, C.-Y.; Zhang, Z.-Z.; Dong, Y.-Y.; Shang, G.-Z.; Zhu, X.-H.; Gao, H.-J.; Wan, X.-C. Removal of fluoride from drinking water using tea waste loaded with Al/Fe oxides: A novel, safe, and efficient biosorbent. Appl. Surf. Sci. 2015, 328, 34–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Liu, L.; Chen, F. Fluoride removal by Fe(III)-loaded ligand exchange cotton cellulose adsorbent from drinking water. Carbohydr. Polym. 2008, 72, 144–150. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Q.; Dong, J.; Cheng, X.; Xu, J. Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe(III). Desalination 2011, 277, 121–127. [Google Scholar] [CrossRef]
- Terasaka, S.; Kamitakahara, M.; Yokoi, T.; Matsubara, H. Effect of carbonate inclusion on fluoride ion removal by hydroxyapatite: A discussion from the viewpoint of hydroxyapatite dissolution. J. Ceram. Soc. Jpn. 2016, 124, 1211–1216. [Google Scholar] [CrossRef] [Green Version]
- Janardhana, C.; Rao, G.N.; Sathish, R.S.; Kumar, P.S.; Kumar, V.A.; Madhav, M.V. Study on defluoridation of drinking water using zirconium ion impregnated activated charcoals. Indian J. Chem. Technol. 2007, 14, 350–354. [Google Scholar]
- Sathish, R.S.; Sairam, S.; Raja, V.G.; Rao, G.N.; Janardhana, C. Defluoridation of Water Using Zirconium Impregnated Coconut Fiber Carbon. Sep. Sci. Technol. 2008, 43, 3676–3694. [Google Scholar] [CrossRef]
- Alagumuthu, G.; Rajan, M. Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nutshell carbon. Chem. Eng. J. 2010, 158, 451–457. [Google Scholar] [CrossRef]
- Dou, X.; Mohan, D.; Pittman, C.U., Jr.; Yang, S. Remediating fluoride from water using hydrous zirconium oxide. Chem. Eng. J. 2012, 198, 236–245. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. A novel ultrasonication method in the preparation of zirconium impregnated cellulose for effective fluoride adsorption. Ultrason. Sonochem. 2014, 21, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Jimenez, L.H.; Hurt, R.H.; Matos, J.; Rangel-Mendez, J.R. Zirconium-Carbon Hybrid Sorbent for Removal of Fluoride from Water: Oxalic Acid Mediated Zr(IV) Assembly and Adsorption Mechanism. Environ. Sci. Technol. 2014, 48, 1166–1174. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Ohgai, M. Effects of ZrOCl2 Concentration and Reaction Temperature on the Formation Process of Hydrous-Zirconia Fine Particles. J. Ceram. Soc. Jpn. 1999, 107, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Morishita, T.; Suzuki, R.; Tsumura, T.; Habazaki, H.; Inagaki, M. Preparation of mesoporous carbons by carbonization of the mixtures of poly(vinyl alcohol) with magnesium salts. TANSO 2006, 223, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Morishita, T.; Ishihara, K.; Kato, M.; Tsumura, T.; Inagaki, M. Mesoporous carbons prepared from mixtures of magnesium citrate with poly(vinyl alcohol). TANSO 2007, 226, 19–24. [Google Scholar] [CrossRef]
- Konno, H.; Onishi, H.; Yoshizawa, N.; Azumi, K. MgO-templated nitrogen-containing carbons derived from different organic compounds for capacitor electrodes. J. Power Source 2010, 195, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Cisar, A.; Corbett, J.D.; Daake, R.L. The Zirconium Dichloride Phase Region. Synthesis, Structure, and Photoelectron Spectral Studies of 3R-ZrCl2, 6T-Zr1.05Cl2, and Related Phases. Inorg. Chem. 1979, 18, 836–843. [Google Scholar] [CrossRef]
- Baba, Y.; Sasaki, T.A. Application of X-ray-induced Auger electron spectroscopy to state analysis of hydrogen implanted in Y, Zr, and Nb metals. Surf. Interf. Anal. 1984, 6, 171–173. [Google Scholar] [CrossRef]
- Kanô, F.; Abe, I.; Kamaya, H.; Ueda, I. Fractal model for adsorption on activated carbon surfaces: Langmuir and Freundlich adsorption. Surf. Sci. 2000, 467, 131–138. [Google Scholar] [CrossRef]





| Product ID | Specific Surface Area1 /m2 g−1 | Mesopore Diameter /nm |
|---|---|---|
| MJ(4)010 | 1100 | 10 |
| MJ(4)030 | 800 | 30 |
| MJ(4)150 | 300 | 150 |
| Adsorbent | Specific Surface Area/m2 g−1 | |
|---|---|---|
| Before Modification | After Modification | |
| ZrO2·xH2O/MJ(4)010 | 1171 | 824 |
| ZrO2·xH2O/MJ(4)030 | 807 | 602 |
| ZrO2·xH2O/MJ(4)150 | 309 | 299 |
| Adsorbent | KF/mg1-1/n g−1 L1/n | KF Enhancement After Modification/Times | |
|---|---|---|---|
| Before Modification | After Modification | ||
| ZrO2·xH2O/MJ(4)010 | 0.044 | 1.68 | 38 |
| ZrO2·xH2O/MJ(4)030 | 0.39 | 3.35 | 8.6 |
| ZrO2·xH2O/MJ(4)150 | 0.53 | 3.03 | 5.7 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, T. Removal of F− from Water Using Templated Mesoporous Carbon Modified with Hydrated Zirconium Oxide. C 2020, 6, 13. https://doi.org/10.3390/c6010013
Takada T. Removal of F− from Water Using Templated Mesoporous Carbon Modified with Hydrated Zirconium Oxide. C. 2020; 6(1):13. https://doi.org/10.3390/c6010013
Chicago/Turabian StyleTakada, Tomoya. 2020. "Removal of F− from Water Using Templated Mesoporous Carbon Modified with Hydrated Zirconium Oxide" C 6, no. 1: 13. https://doi.org/10.3390/c6010013
APA StyleTakada, T. (2020). Removal of F− from Water Using Templated Mesoporous Carbon Modified with Hydrated Zirconium Oxide. C, 6(1), 13. https://doi.org/10.3390/c6010013