Impact of Nitrogen, Boron and Phosphorus Impurities on the Electronic Structure of Diamond Probed by X-ray Spectroscopies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diamond Materials
2.2. Secondary Ion Mass Spectrometry Analysis
2.3. Differential Interference Contrast Microscopy
2.4. X-ray Absorption Spectroscopy
2.5. X-ray Emission and Photoemission Spectroscopies
3. Results
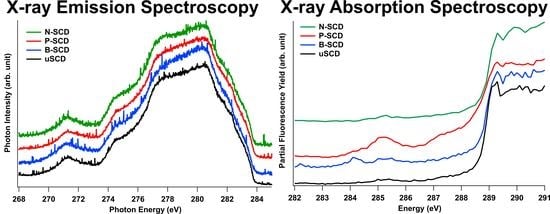
3.1. Electronic States of Diamond Probed by XAS and XES at the C K-Edge
3.2. Unoccupied Electronic States of Doped Diamond
3.3. Occupied Electronic States of Doped Diamond
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, D.; Zhang, L.; Ruther, R.E.; Hamers, R.J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 2013, 12, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, D.; Nathanson, G.M.; Hamers, R.J. Selective Photoelectrochemical Reduction of Aqueous CO2 to CO by Solvated Electrons. Angew. Chem. Int. Ed. 2014, 53, 9746–9750. [Google Scholar] [CrossRef]
- Choudhury, S.; Kiendl, B.; Ren, J.; Gao, F.; Knittel, P.; Nebel, C.; Venerosy, A.; Girard, H.; Arnault, J.-C.; Krueger, A.; et al. Combining nanostructuration with boron doping to alter sub band gap acceptor states in diamond materials. J. Mater. Chem. A 2018, 6, 16645–16654. [Google Scholar] [CrossRef] [Green Version]
- Knittel, P.; Buchner, F.; Hadzifejzovic, E.; Giese, C.; Quellmalz, P.; Seidel, R.; Petit, T.; Iliev, B.; Schubert, T.J.S.; Nebel, C.E.; et al. Nanostructured Boron Doped Diamond Electrodes with Increased Reactivity for Solar-Driven CO2 Reduction in Room Temperature Ionic Liquids. ChemCatChem 2020, 12, 5548–5557. [Google Scholar] [CrossRef]
- Zegkinoglou, I.; Cook, P.L.; Johnson, P.S.; Yang, W.; Guo, J.; Pickup, D.; González-Moreno, R.; Rogero, C.; Ruther, R.E.; Rigsby, M.L.; et al. Electronic Structure of Diamond Surfaces Functionalized by Ru(tpy)2. J. Phys. Chem. C 2012, 116, 13877–13883. [Google Scholar] [CrossRef]
- Yeap, W.S.; Liu, X.; Bevk, D.; Pasquarelli, A.; Lutsen, L.; Fahlman, M.; Maes, W.; Haenen, K. Functionalization of Boron-Doped Nanocrystalline Diamond with N3 Dye Molecules. ACS Appl. Mater. Interfaces 2014, 6, 10322–10329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Bandy, J.A.; Hamers, R.J. Enhanced Photocatalytic Activity of Diamond Thin Films Using Embedded Ag Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 5395–5403. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Koeck, F.A.M.; Zhu, C.; Nemanich, R.J. Combined visible light photo-emission and low temperature thermionic emission from nitrogen doped diamond films. Appl. Phys. Lett. 2011, 99, 202101. [Google Scholar] [CrossRef]
- Zhu, W.; Kochanski, G.P.; Jin, S.; Seibles, L. Defect-enhanced electron field emission from chemical vapor deposited diamond. J. Appl. Phys. 1995, 78, 2707–2711. [Google Scholar] [CrossRef]
- Geis, M.W.; Twichell, J.C.; Efremow, N.N.; Krohn, K.; Lyszczarz, T.M. Comparison of electric field emission from nitrogen-doped, type Ib diamond, and boron-doped diamond. Appl. Phys. Lett. 1996, 68, 2294–2296. [Google Scholar] [CrossRef]
- Lacher, F.; Wild, C.; Behr, D.; Koidl, P. Electron field emission from thin fine-grained CVD diamond films. Diam. Relat. Mater. 1997, 6, 1111–1116. [Google Scholar] [CrossRef]
- Yamada, T.; Okano, K.; Yamaguchi, H.; Kato, H.; Shikata, S.-I.; Nebel, C.E. Field emission from reconstructed heavily phosphorus-doped homoepitaxial diamond (111). Appl. Phys. Lett. 2006, 88, 212114. [Google Scholar] [CrossRef]
- Kajihara, S.A.; Antonelli, A.; Bernholc, J.; Car, R. Nitrogen and potential n-type dopants in diamond. Phys. Rev. Lett. 1991, 66, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Mort, J.; Machonkin, M.A.; Okumura, K. Compensation effects in nitrogen-doped diamond thin films. Appl. Phys. Lett. 1991, 59, 3148–3150. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, D.; Ogura, M.; Yamada, T.; Kataoka, M.; Kimura, Y.; Sobue, S.; Nebel, C.E.; Yamasaki, S. Heavily phosphorus-doped nano-crystalline diamond electrode for thermionic emission application. Diam. Relat. Mater. 2016, 63, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Grotjohn, T.; Tran, D.; Yaran, M.; Demlow, S.N.; Schuelke, T. Heavy phosphorus doping by epitaxial growth on the (111) diamond surface. Diam. Relat. Mater. 2014, 44, 129–133. [Google Scholar] [CrossRef]
- De Groot, F.; Kotani, A. Core Level Spectroscopy of Solids; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. IX. Data for 41 elemental solids over the 50 eV to 30 keV range. Surf. Interface Anal. 2011, 43, 689–713. [Google Scholar] [CrossRef]
- Henke, B.; Gullikson, E.; Davis, J. X-Ray Interactions: Photoabsorption, Scattering, Transmission, and Reflection at E = 50-30,000 eV, Z = 1–92. At. Data Nucl. Data Tables 1993, 54, 181–342. [Google Scholar] [CrossRef] [Green Version]
- Füner, M.; Wild, C.; Koidl, P. Novel microwave plasma reactor for diamond synthesis. Appl. Phys. Lett. 1998, 72, 1149–1151. [Google Scholar] [CrossRef]
- Schulz, C.; Lieutenant, K.; Xiao, J.; Hofmann, T.; Wong, D.; Habicht, K. Characterization of the soft X-ray spectrometer PEAXIS at BESSY II. J. Synchrotron Radiat. 2020, 27, 238–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morar, J.F.; Himpsel, F.J.; Hollinger, G.; Hughes, G.; Jordan, J.L. Observation of a C- 1s Core Exciton in Diamond. Phys. Rev. Lett. 1985, 54, 1960–1963. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Koizumi, S.; Otsuka, T.; Suhara, M.; Morohasi, T.; Kurmaev, E.Z.; Chong, D.P. Analysis of XPS and XES of diamond and graphite by DFT calculations using model molecules. J. Comput. Chem. 2000, 22, 102–108. [Google Scholar] [CrossRef]
- Graupner, R.; Ristein, J.; Ley, L.; Jung, C. Surface-sensitive K-edge absorption spectroscopy on clean and hydrogen-terminated diamond (111) and (100) surfaces. Phys. Rev. B 1999, 60, 17023–17029. [Google Scholar] [CrossRef]
- Birrell, J.; Gerbi, J.E.; Auciello, O.; Gibson, J.M.; Gruen, D.M.; Carlisle, J.A. Bonding structure in nitrogen doped ultrananocrystalline diamond. J. Appl. Phys. 2003, 93, 5606–5612. [Google Scholar] [CrossRef]
- Haenen, K.; Nesládek, M.; De Schepper, L.; Kravets, R.; Vaněček, M.; Koizumi, S. The phosphorous level fine structure in homoepitaxial and polycrystalline n-type CVD diamond. Diam. Relat. Mater. 2004, 13, 2041–2045. [Google Scholar] [CrossRef]
- Alfieri, G.; Kranz, L.; Mihaila, A. Phosphorus-Related Complexes and Shallow Doping in Diamond. Phys. Status Solidi (RRL) Rapid Res. Lett. 2018, 12, 1700409. [Google Scholar] [CrossRef]
- Nakamura, J.; Kabasawa, E.; Yamada, N.; Einaga, Y.; Saito, D.; Isshiki, H.; Yugo, S.; Perera, R.C.C. Electronic structures of B2p and C2p levels in boron-doped diamond films studied using soft X-ray absorption and emission spectroscopy. Phys. Rev. B 2004, 70, 245111. [Google Scholar] [CrossRef] [Green Version]
- Shikata, S.; Yamaguchi, K.; Fujiwara, A.; Tamenori, Y.; Tsuruta, K.; Yamada, T.; Nicley, S.S.; Haenen, K.; Koizumi, S. X-ray absorption near edge structure and extended X-ray absorption fine structure studies of P doped (111) diamond. Diam. Relat. Mater. 2020, 105, 107769. [Google Scholar] [CrossRef]
- Francz, G.; Oelhafen, P. Valence band spectroscopy of reconstructed (100) and (111) natural diamond. Diam. Relat. Mater. 1995, 4, 539–543. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, S.; Golnak, R.; Schulz, C.; Lieutenant, K.; Tranchant, N.; Arnault, J.-C.; Pinault-Thaury, M.-A.; Jomard, F.; Knittel, P.; Petit, T. Impact of Nitrogen, Boron and Phosphorus Impurities on the Electronic Structure of Diamond Probed by X-ray Spectroscopies. C 2021, 7, 28. https://doi.org/10.3390/c7010028
Choudhury S, Golnak R, Schulz C, Lieutenant K, Tranchant N, Arnault J-C, Pinault-Thaury M-A, Jomard F, Knittel P, Petit T. Impact of Nitrogen, Boron and Phosphorus Impurities on the Electronic Structure of Diamond Probed by X-ray Spectroscopies. C. 2021; 7(1):28. https://doi.org/10.3390/c7010028
Chicago/Turabian StyleChoudhury, Sneha, Ronny Golnak, Christian Schulz, Klaus Lieutenant, Nicolas Tranchant, Jean-Charles Arnault, Marie-Amandine Pinault-Thaury, François Jomard, Peter Knittel, and Tristan Petit. 2021. "Impact of Nitrogen, Boron and Phosphorus Impurities on the Electronic Structure of Diamond Probed by X-ray Spectroscopies" C 7, no. 1: 28. https://doi.org/10.3390/c7010028
APA StyleChoudhury, S., Golnak, R., Schulz, C., Lieutenant, K., Tranchant, N., Arnault, J. -C., Pinault-Thaury, M. -A., Jomard, F., Knittel, P., & Petit, T. (2021). Impact of Nitrogen, Boron and Phosphorus Impurities on the Electronic Structure of Diamond Probed by X-ray Spectroscopies. C, 7(1), 28. https://doi.org/10.3390/c7010028