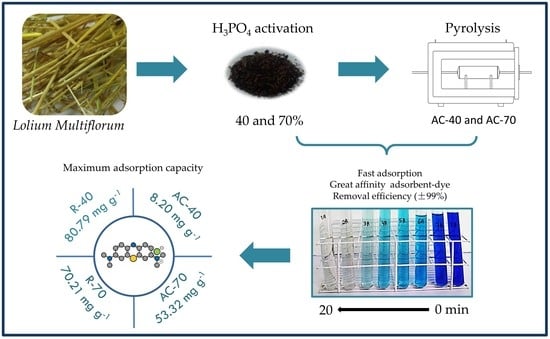
Application of Lolium multiflorum as an Efficient Raw Material in the Production of Adsorbent for Removal of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Adsorbents
2.2. Adsorption Experiments
3. Results
3.1. Characterization of Lolium multiflorum and Adsorbents
3.2. Adsorption Experiments
3.3. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Aboul-Enein, H.Y. Instrumental Methods in Metal Ion Speciation; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429133350. [Google Scholar]
- dos Santos, K.J.L.; de Souza dos Santos, G.E.; de Sá, Í.M.G.L.; de Carvalho, S.H.V.; Soletti, J.I.; Meili, L.; da Silva Duarte, J.L.; Bispo, M.D.; Dotto, G.L. Syagrus Oleracea–Activated Carbon Prepared by Vacuum Pyrolysis for Methylene Blue Adsorption. Environ. Sci. Pollut. Res. 2019, 26, 16470–16481. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Khan, M.M.; Gracia, F.; Qin, J.; Gupta, V.K.; Arumainathan, S. Ce3+-Ion-Induced Visible-Light Photocatalytic Degradation and Electrochemical Activity of ZnO/CeO2 Nanocomposite. Sci. Rep. 2016, 6, 31641. [Google Scholar] [CrossRef] [PubMed]
- El-Zawahry, M.M.; Abdelghaffar, F.; Abdelghaffar, R.A.; Hassabo, A.G. Equilibrium and Kinetic Models on the Adsorption of Reactive Black 5 from Aqueous Solution Using Eichhornia Crassipes/Chitosan Composite. Carbohydr. Polym. 2016, 136, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Zakaria, M.; Mohd.-Setapar, S.H.; Che-Yunus, M.A. Sludge-Adsorbents from Palm Oil Mill Effluent for Methylene Blue Removal. J. Environ. Chem. Eng. 2013, 1, 1091–1098. [Google Scholar] [CrossRef]
- Gobi, K.; Mashitah, M.D.; Vadivelu, V.M. Adsorptive Removal of Methylene Blue Using Novel Adsorbent from Palm Oil Mill Effluent Waste Activated Sludge: Equilibrium, Thermodynamics and Kinetic Studies. Chem. Eng. J. 2011, 171, 1246–1252. [Google Scholar] [CrossRef]
- Chahm, T.; Martins, B.A.; Rodrigues, C.A. Adsorption of Methylene Blue and Crystal Violet on Low-Cost Adsorbent: Waste Fruits of Rapanea Ferruginea (Ethanol-Treated and H2SO4-Treated). Environ. Earth Sci. 2018, 77, 508. [Google Scholar] [CrossRef]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck Netto, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of Congo Red and Methylene Blue Dyes on an Ashitaba Waste and a Walnut Shell-Based Activated Carbon from Aqueous Solutions: Experiments, Characterization and Physical Interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch Adsorption of Basic Dye Using Acid Treated Kenaf Fibre Char: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2012, 181–182, 449–457. [Google Scholar] [CrossRef]
- Ghaedi, M.; Heidarpour, S.; Nasiri Kokhdan, S.; Sahraie, R.; Daneshfar, A.; Brazesh, B. Comparison of Silver and Palladium Nanoparticles Loaded on Activated Carbon for Efficient Removal of Methylene Blue: Kinetic and Isotherm Study of Removal Process. Powder Technol. 2012, 228, 18–25. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Wawrzkiewicz, M.; Wiśniewska, M.; Wołowicz, A.; Gun’ko, V.M.; Zarko, V.I. Mixed Silica-Alumina Oxide as Sorbent for Dyes and Metal Ions Removal from Aqueous Solutions and Wastewaters. Microporous Mesoporous Mater. 2017, 250, 128–147. [Google Scholar] [CrossRef]
- Dotto, L.G.; Salau, N.P.G.; Picin, J.S.; Cadaval, T.R.S., Jr.; De Pinto, L.A.A. Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; p. 256. [Google Scholar]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Fundamentals of Adsorption Technology. In Interface Science and Technology; Mehrorang Ghaedi, Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–70. [Google Scholar]
- Shahmoradi Ghaheh, F.; Taghizadeh, M.; Taghizadeh, A.; Hayati, B.; Mahmoodi, N.M.; Parastar, S. Clean Synthesis of Rock Candy-like Metal–Organic Framework Biocomposite for Toxic Contaminants Remediation. Environ. Technol. Innov. 2021, 23, 101747. [Google Scholar] [CrossRef]
- Wamba, A.G.N.; Ndi, S.K.; Lima, E.C.; Kayem, J.G.; Thue, P.S.; Costa, T.M.H.; Quevedo, A.B.; Benvenutti, E.V.; Machado, F.M. Preparation, Characterization of Titanate Nanosheet–Pozzolan Nanocomposite and Its Use as an Adsorbent for Removal of Diclofenac from Simulated Hospital Effluents. J. Taiwan Inst. Chem. Eng. 2019, 102, 321–329. [Google Scholar] [CrossRef]
- Liew, R.K.; Chai, C.; Yek, P.N.Y.; Phang, X.Y.; Chong, M.Y.; Nam, W.L.; Su, M.H.; Lam, W.H.; Ma, N.L.; Lam, S.S. Innovative Production of Highly Porous Carbon for Industrial Effluent Remediation via Microwave Vacuum Pyrolysis plus Sodium-Potassium Hydroxide Mixture Activation. J. Clean. Prod. 2019, 208, 1436–1445. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of Methylene Blue from Aqueous Solution by Sewage Sludge-Derived Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics and Mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Seid-Mohammadi, A.; Asgarai, G.; Ghorbanian, Z.; Dargahi, A. The Removal of Cephalexin Antibiotic in Aqueous Solutions by Ultrasonic Waves/Hydrogen Peroxide/Nickel Oxide Nanoparticles (US/H2O2/NiO) Hybrid Process. Sep. Sci. Technol. 2020, 55, 1558–1568. [Google Scholar] [CrossRef]
- Asuha, S.; Fei, F.; Wurendaodi, W.; Zhao, S.; Wu, H.; Zhuang, X. Activation of Kaolinite by a Low-Temperature Chemical Method and Its Effect on Methylene Blue Adsorption. Powder Technol. 2020, 361, 624–632. [Google Scholar] [CrossRef]
- Ren, C.; Guo, D.; Liu, X.; Li, R.; Zhang, Z. Performance of the Emerging Biochar on the Stabilization of Potentially Toxic Metals in Smelter- and Mining-Contaminated Soils. Environ. Sci. Pollut. Res. 2020, 27, 43428–43438. [Google Scholar] [CrossRef]
- Imran, M.; Islam, A.; Farooq, M.U.; Ye, J.; Zhang, P. Characterization and Adsorption Capacity of Modified 3D Porous Aerogel from Grapefruit Peels for Removal of Oils and Organic Solvents. Environ. Sci. Pollut. Res. 2020, 27, 43493–43504. [Google Scholar] [CrossRef]
- Park, M.H.; Jeong, S.; Kim, J.Y. Adsorption of NH3-N onto Rice Straw-Derived Biochar. J. Environ. Chem. Eng. 2019, 7, 103039. [Google Scholar] [CrossRef]
- Rovani, S.; Rodrigues, A.G.; Medeiros, L.F.; Cataluña, R.; Lima, É.C.; Fernandes, A.N. Synthesis and Characterisation of Activated Carbon from Agroindustrial Waste—Preliminary Study of 17β-Estradiol Removal from Aqueous Solution. J. Environ. Chem. Eng. 2016, 4, 2128–2137. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of Activated Carbons Prepared from Wheat Straw via ZnCl2 and KOH Activation. Waste Biomass Valorization 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Fontaneli, R.S.; Santos, H.P.; Fontaneli, R.S. Forrageiras Para Integração Lavoura-Pecuária-Floresta Na Região Sul-Brasileira, 2nd ed.; Renato Serena Fontaneli, H.P., dos Santos, R.S.F., Eds.; Embrapa: Brasília, Brazil, 2012; ISBN 9788570351043. [Google Scholar]
- Silva, E.O.; Santos, V.D.; Araujo, E.B.; Guterres, F.P.; Zottis, R.; Flores, W.H.; Almeida, A.R.F. Removal of Methylene Blue from Aqueous Solution by Ryegrass Straw. Int. J. Environ. Sci. Technol. 2020, 17, 3723–3740. [Google Scholar] [CrossRef]
- Cunniff, P.; AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- ASTM International. ASTM D 1762-84: Standard Test Method for Chemical Analysis of Wood Charcoal; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM International. ASTM E872-82: Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM International. ASTM E1755: Standard Test Method for Ash in Biomass; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Lagergren, S.Y. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Für Chem. Und Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Lopes, E.C.N.; Dos Anjos, F.S.C.; Vieira, E.F.S.; Cestari, A.R. An Alternative Avrami Equation to Evaluate Kinetic Parameters of the Interaction of Hg(II) with Thin Chitosan Membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Tóth, J. State Equations of the Solid-Gas Interface Layers. Acta Chim. (Acad. Sci. Hung.) 1971, 69, 311–328. [Google Scholar]
- Silgado, K.J.; Marrugo, G.D.; Puello, J. Adsorption of Chromium (VI) by Activated Carbon Produced from Oil Palm Endocarp. Chem. Eng. Trans. 2014, 37, 721–726. [Google Scholar] [CrossRef]
- Puchana-Rosero, M.J.; Adebayo, M.A.; Lima, E.C.; Machado, F.M.; Thue, P.S.; Vaghetti, J.C.P.; Umpierres, C.S.; Gutterres, M. Microwave-Assisted Activated Carbon Obtained from the Sludge of Tannery-Treatment Effluent Plant for Removal of Leather Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 105–115. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Daud, W.M.A.B.W.; Krishnaiah, D.; Yee, H.S. Preparation and Characterization of Activated Carbon from Typha Orientalis Leaves. Int. J. Ind. Chem. 2015, 6, 9–21. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Sae-Ung, S.; Tanthapanichakoon, W. Preparation of Activated Carbons from Coffee Residue for the Adsorption of Formaldehyde. Sep. Purif. Technol. 2005, 42, 159–168. [Google Scholar] [CrossRef]
- Valério Filho, A.; Xavaré Kulman, R.; Vaz Tholozan, L.; Felkl de Almeida, A.R.; Silveira da Rosa, G. Preparation and Characterization of Activated Carbon Obtained from Water Treatment Plant Sludge for Removal of Cationic Dye from Wastewater. Processes 2020, 8, 1549. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. HEMP-Derived Activated Carbon Fibers by Chemical Activation with Phosphoric Acid. Fuel 2009, 88, 19–26. [Google Scholar] [CrossRef]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Multivariate Statistical Analysis of X-Ray Data from Cellulose: A New Method to Determine Degree of Crystallinity and Predict Hydrolysis Rates. Bioresour. Technol. 2010, 101, 4461–4471. [Google Scholar] [CrossRef]
- Wassie, A.B.; Srivastava, V.C. Chemical Treatment of Teff Straw by Sodium Hydroxide, Phosphoric Acid and Zinc Chloride: Adsorptive Removal of Chromium. Int. J. Environ. Sci. Technol. 2016, 13, 2415–2426. [Google Scholar] [CrossRef]
- Filho, A.V.; Kulman, R.X.; Janner, N.N.; Tholozan, L.V.; de Almeida, A.R.F.; da Rosa, G.S. Optimization of Cationic Dye Removal Using a High Surface Area-Activated Carbon from Water Treatment Sludge. Bull. Mater. Sci. 2021, 44, 41. [Google Scholar] [CrossRef]
- Gao, C.; Xiong, G.Y.; Luo, H.L.; Ren, K.J.; Huang, Y.; Wan, Y.Z. Dynamic Interaction between the Growing Ca-P Minerals and Bacterial Cellulose Nanofibers during Early Biomineralization Process. Cellulose 2010, 17, 365–373. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Santos, P.L.; Bonacin, J.A.; Passos, R.R.; Pocrifka, L.A. Rice Husk Reuse in the Preparation of SnO2/SiO2Nanocomposite. Mater. Res. 2015, 18, 639–643. [Google Scholar] [CrossRef]
- Zuo, R.F.; Du, G.X.; Yang, W.G.; Liao, L.B.; Li, Z. Mineralogical and Chemical Characteristics of a Powder and Purified Quartz from Yunnan Province. Open Geosci. 2016, 8, 606–611. [Google Scholar] [CrossRef]
- Lima, D.R.; Sellaoui, L.; Klein, L.; Reis, G.S.; Lima, É.C.; Dotto, G.L. Physicochemical and Thermodynamic Study of Malachite Green Adsorption on Raw and Modified Corn Straw. Can. J. Chem. Eng. 2018, 96, 779–787. [Google Scholar] [CrossRef]
- Valério Filho, A.; Tholozan, L.V.; da Silva, E.O.; Meili, L.; de Almeida, A.R.F.; da Rosa, G.S. Perspectives of the Reuse of Agricultural Wastes from the Rio Grande Do Sul, Brazil, as New Adsorbent Materials. In Biomass-Derived Materials for Environmental Applications; Anastopoulos, I., Lima, E.C., Meili, L., Giannakoudakis, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–266. ISBN 9780323919142. [Google Scholar]
- Raupp, Í.N.; Valério Filho, A.; Arim, A.L.; Muniz, A.R.C.; da Rosa, G.S. Development and Characterization of Activated Carbon from Olive Pomace: Experimental Design, Kinetic and Equilibrium Studies in Nimesulide Adsorption. Materials 2021, 14, 6820. [Google Scholar] [CrossRef]
- Bortoluz, J.; Ferrarini, F.; Bonetto, L.R.; da Silva Crespo, J.; Giovanela, M. Use of Low-Cost Natural Waste from the Furniture Industry for the Removal of Methylene Blue by Adsorption: Isotherms, Kinetics and Thermodynamics. Cellulose 2020, 27, 6445–6466. [Google Scholar] [CrossRef]
- Tholozan, L.V.; Valério Filho, A.; Maron, G.K.; Carreno, N.L.V.; da Rocha, C.M.; Bordin, J.; da Rosa, G.S. Sphagnum Perichaetiale Hampe Biomass as a Novel, Green, and Low-Cost Biosorbent in the Adsorption of Toxic Crystal Violet Dye. Environ. Sci. Pollut. Res. 2023, 30, 52472–52484. [Google Scholar] [CrossRef]
- Filho, A.C.D.; Mazzocato, A.C.; Dotto, G.L.; Thue, P.S.; Pavan, F.A. Eragrostis Plana Nees as a Novel Eco-Friendly Adsorbent for Removal of Crystal Violet from Aqueous Solutions. Environ. Sci. Pollut. Res. 2017, 24, 19909–19919. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Netto, M.S.; Georgin, J.; Luiz Dotto, G.; Abu Shayeb, M.K.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Preparation and Characterization of a Novel Mountain Soursop Seeds Powder Adsorbent and Its Application for the Removal of Crystal Violet and Methylene Blue from Aqueous Solutions. Chem. Eng. J. 2020, 391, 123617. [Google Scholar] [CrossRef]
- Cimirro, N.F.G.M.; Lima, E.C.; Cunha, M.R.; Dias, S.L.P.; Thue, P.S.; Mazzocato, A.C.; Dotto, G.L.; Gelesky, M.A.; Pavan, F.A. Removal of Pharmaceutical Compounds from Aqueous Solution by Novel Activated Carbon Synthesized from Lovegrass (Poaceae). Environ. Sci. Pollut. Res. 2020, 27, 21442–21454. [Google Scholar] [CrossRef] [PubMed]
- Salomón, Y.L.O.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Foletto, E.L.; Allasia, D.; Dotto, G.L. Application of Seed Residues from Anadenanthera Macrocarpa and Cedrela Fissilis as Alternative Adsorbents for Remarkable Removal of Methylene Blue Dye in Aqueous Solutions. Environ. Sci. Pollut. Res. 2021, 28, 2342–2354. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Fagundes, J.L.S.; Georgin, J.; Salau, N.P.G.; Dotto, G.L. A Mass Transfer Study Considering Intraparticle Diffusion and Axial Dispersion for Fixed-Bed Adsorption of Crystal Violet on Pecan Pericarp (Carya Illinoensis). Chem. Eng. J. 2020, 397, 125423. [Google Scholar] [CrossRef]
- Salomón, Y.L.D.O.; Georgin, J.; Reis, G.; Lima, E.C.; Oliveira, M.L.S.; Franco, D.S.P.; Netto, M.S.; Allasia, D.; Dotto, G.L. Utilization of Pacara Earpod Tree (Enterolobium Contortisilquum) and Ironwood (Caesalpinia Leiostachya) Seeds as Low-Cost Biosorbents for Removal of Basic Fuchsin. Environ. Sci. Pollut. Res. 2020, 27, 33307–33320. [Google Scholar] [CrossRef] [PubMed]
- Salomón, Y.L.D.O.; Georgin, J.; Franco, D.S.; Netto, M.S.; Piccilli, D.G.; Foletto, E.L.; Oliveira, L.F.; Dotto, G.L. High-Performance Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide in Water Using Activated Carbon Derived from Queen Palm Fruit Endocarp (Syagrus Romanzoffiana). J. Environ. Chem. Eng. 2021, 9, 104911. [Google Scholar] [CrossRef]
- Yacob, A.R.; Al Swaidan, H.M. Phosphoric Acid Effect on Prepared Activated Carbon from Saudi Arabia’s Date Frond Waste. Appl. Mech. Mater. 2012, 110–116, 2124–2130. [Google Scholar] [CrossRef]
- Macedo, J.D.S.; Júnior, N.B.D.C.; Almeida, L.E.; Vieira, E.F.D.S.; Cestari, A.R.; Gimenez, I.D.F.; Carreño, N.L.V.; Barreto, L.S. Kinetic and Calorimetric Study of the Adsorption of Dyes on Mesoporous Activated Carbon Prepared from Coconut Coir Dust. J. Colloid Interface Sci. 2006, 298, 515–522. [Google Scholar] [CrossRef]
- Fontana, K.B.; Chaves, E.S.; Sanchez, J.D.S.; Watanabe, E.R.L.R.; Pietrobelli, J.M.T.A.; Lenzi, G.G. Textile Dye Removal from Aqueous Solutions by Malt Bagasse: Isotherm, Kinetic and Thermodynamic Studies. Ecotoxicol. Environ. Saf. 2016, 124, 329–336. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Alves, C.C.O.; Franca, A.S.; Oliveira, L.S. Comparison of Microwave Assisted Thermo-Chemical Procedures in the Production of Adsorbents for Wastewater Treatment. Int. J. Environ. Sci. Dev. 2015, 6, 888–894. [Google Scholar] [CrossRef]
- Xie, X.; Gao, H.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z. Polyethyleneimine Modified Activated Carbon for Adsorption of Cd(II) in Aqueous Solution. J. Environ. Chem. Eng. 2019, 7, 103183. [Google Scholar] [CrossRef]
- Kumar, P.; Chauhan, M.S. Adsorption of Chromium (VI) from the Synthetic Aqueous Solution Using Chemically Modified Dried Water Hyacinth Roots. J. Environ. Chem. Eng. 2019, 7, 103218. [Google Scholar] [CrossRef]
- Valério Filho, A.; Tholozan, L.V.; Arim, A.L.; de Almeida, A.R.F.; da Rosa, G.S. High-Performance Removal of Anti-Inflammatory Using Activated Carbon from Water Treatment Plant Sludge: Fixed-Bed and Batch Studies. Int. J. Environ. Sci. Technol. 2023, 20, 3633–3644. [Google Scholar] [CrossRef]
- Archin, S.; Sharifi, S.H.; Asadpour, G. Optimization and Modeling of Simultaneous Ultrasound-Assisted Adsorption of Binary Dyes Using Activated Carbon from Tobacco Residues: Response Surface Methodology. J. Clean. Prod. 2019, 239, 118136. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of Methylene Blue on Activated Carbon Produced from Flamboyant Pods (Delonix Regia): Study of Adsorption Isotherms and Kinetic Models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; da Cunha, B.M.; Cardoso, N.F.; Brasil, J.L.; Dias, S.L.P. Pecan Nutshell as Biosorbent to Remove Cu(II), Mn(II) and Pb(II) from Aqueous Solutions. J. Hazard. Mater. 2009, 162, 270–280. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Vaghetti, J.C.P.; Dias, S.L.P.; Pavan, F.A. Application of Carbon Adsorbents Prepared from Brazilian-Pine Fruit Shell for the Removal of Reactive Orange 16 from Aqueous Solution: Kinetic, Equilibrium, and Thermodynamic Studies. J. Environ. Manag. 2010, 91, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.C.O.; Franca, A.S.; Oliveira, L.S. Removal of Phenylalanine from Aqueous Solutions with Thermo-Chemically Modified Corn Cobs as Adsorbents. LWT-Food Sci. Technol. 2013, 51, 1–8. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Adebayo, M.A.; Sampaio, C.H.; Lima, E.C.; Thue, P.S.; de Brum, I.A.S.; Dias, S.L.P.; Pavan, F.A. Removal of Phenolic Compounds from Aqueous Solutions Using Sludge-Based Activated Carbons Prepared by Conventional Heating and Microwave-Assisted Pyrolysis. Water Air Soil Pollut. 2017, 228, 33. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 846, 3973. [Google Scholar] [CrossRef]
- Amiri, M.K.; Ghaemi, A.; Arjomandi, H. Experimental, Kinetics and Isothermal Modeling of Carbon Dioxide Adsorption with 13X Zeolite in a Fixed Bed Column. Iran. J. Chem. Eng. 2019, 16, 54–64. [Google Scholar]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-Activated Carbon of High Surface Area Produced from Coconut Shell: Kinetics and Equilibrium Studies from the Methylene Blue Adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Ramezani, H.; Azizi, S.N.; Cravotto, G. Improved Removal of Methylene Blue on Modified Hierarchical Zeolite Y: Achieved by a “Destructive-Constructive” Method. Green Process. Synth. 2019, 8, 730–741. [Google Scholar] [CrossRef]
- Brion-Roby, R.; Gagnon, J.; Nosrati, S.; Deschênes, J.S.; Chabot, B. Adsorption and Desorption of Molybdenum(VI) in Contaminated Water Using a Chitosan Sorbent. J. Water Process Eng. 2018, 23, 13–19. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Lata, S.; Balasubramanian, P. Biosorption Characteristics of Methylene Blue and Malachite Green from Simulated Wastewater onto Carica Papaya Wood Biosorbent. Surf. Interfaces 2018, 10, 197–215. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-Factionalized Biomass Material for Methylene Blue Dye Removal: A Comprehensive Adsorption and Mechanism Study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Porous Structure and Surface Chemistry of Phosphoric Acid Activated Carbon from Corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Palaniyappan, M.; Priyadharshini, M.; Vignesh, A.M.; Thanjiappan, A.; Sebastina Anne Fernando, P.; Tanvir Ahmed, R.; Srinath, R. Adsorption of Basic Dye onto Raw and Surface-Modified Agricultural Waste. Environ. Prog. Sustain. Energy 2014, 33, 87–98. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M.; Bhila, B.; Moloto, H.; Naidoo, E.B. Lead Ions and Methylene Blue Dye Removal from Aqueous Solution by Mucuna Beans (Velvet Beans) Adsorbents. J. Environ. Chem. Eng. 2020, 8, 103557. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Akrout, H.; Assadi, A.A.; Bousselmi, L. Optimization of a Cationic Dye Removal by a Chemically Modified Agriculture By-Product Using Response Surface Methodology: Biomasses Characterization and Adsorption Properties. Environ. Sci. Pollut. Res. 2017, 24, 9831–9846. [Google Scholar] [CrossRef]
- Lata, H.; Garg, V.K.; Gupta, R.K. Removal of a Basic Dye from Aqueous Solution by Adsorption Using Parthenium Hysterophorus: An Agricultural Waste. Dye. Pigment. 2007, 74, 653–658. [Google Scholar] [CrossRef]
- Lima, J.P.; Alvarenga, G.; Goszczynski, A.C.F.; Rosa, G.R.; Lopes, T.J. Batch Adsorption of Methylene Blue Dye Using Enterolobium Contortisiliquum as Bioadsorbent: Experimental, Mathematical Modeling and Simulation. J. Ind. Eng. Chem. 2020, 91, 362–371. [Google Scholar] [CrossRef]
- Yadav, V.; Ali, J.; Garg, M.C. Biosorption of Methylene Blue Dye from Textile-Industry Wastewater onto Sugarcane Bagasse: Response Surface Modeling, Isotherms, Kinetic and Thermodynamic Modeling. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020067. [Google Scholar] [CrossRef]
- Karagöz, S.; Tay, T.; Ucar, S.; Erdem, M. Activated Carbons from Waste Biomass by Sulfuric Acid Activation and Their Use on Methylene Blue Adsorption. Bioresour. Technol. 2008, 99, 6214–6222. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Uma; Upadhyay, S.N. Removal of a Cationic Dye from Wastewaters by Adsorption on Activated Carbon Developed from Coconut Coir. Energy Fuels 2009, 23, 2983–2988. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of Dyes on Activated Carbon Prepared from Apricot Stones and Commercial Activated Carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magnetized Tectona Grandis Sawdust as a Novel Adsorbent: Preparation, Characterization, and Utilization for the Removal of Methylene Blue from Aqueous Solution. Cellulose 2020, 27, 2613–2635. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Sweleh, A.O. Optimizing Textile Dye Removal by Activated Carbon Prepared from Olive Stones. Environ. Technol. Innov. 2019, 16, 100488. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption Mechanism of Methylene Blue from Aqueous Solution onto White Pine (Pinus Durangensis) Sawdust: Effect of Operating Conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R. Soybean Hulls as a Low-cost Biosorbent for Removal of Methylene Blue Contaminant. Environ. Prog. Sustain. Energy 2020, 39, e13328. [Google Scholar] [CrossRef]
- Manna, S.; Roy, D.; Saha, P.; Gopakumar, D.; Thomas, S. Rapid Methylene Blue Adsorption Using Modified Lignocellulosic Materials. Process Saf. Environ. Prot. 2017, 107, 346–356. [Google Scholar] [CrossRef]










| Adsorbents | SBET (m2 g−1) | PV (cm3 g−1) | PD (nm) | Y (%) |
|---|---|---|---|---|
| AC-40 | 14.50 | 0.0252 | 3.73 | 47.60 |
| AC-70 | 68.28 | 0.0603 | 3.25 | 50.33 |
| Adsorbents from Biomass | Chemical Activation | C0 (mg L−1) | Temperature (°C) | Contact Time (h) | mads/Vsol (g L−1) | pH | qmax (mg g−1) |
|---|---|---|---|---|---|---|---|
| R-40 | H3PO4 | 25–360 | 25 | 2 | 4 | 7 | 80.79 |
| R-70 | H3PO4 | 25–360 | 25 | 2 | 4 | 7 | 70.21 |
| AC-40 | H3PO4 | 25–150 | 25 | 2 | 20 | 7 | 8.20 |
| AC-70 | H3PO4 | 50–1000 | 25 | 2 | 20 | 7 | 53.32 |
| LM [28] | - | 150 | - | 2 | 4–32 | 7 | 28.70 |
| LM + NaOH [28] | NaOH | 150 | - | 1 | 4–32 | 67.19 | |
| Carica papaya wood [85] | - | 10–50 | - | 2.3 | 2 | 10 | 32.25 |
| Coconut shell [86] | H2SO4 | 25–200 | 30 | 3 | 1 | 8 | 50.60 |
| AC—Corncob [87] | H3PO4 | 300–1500 | - | 5 | 2 | - | 112.00 |
| Mango seedkernel poder [88] | H2SO4 | 100 | 30 | 0.5 | 4 | 8 | 58.08 |
| Mucuma beans [89] | HCl + HNO3 | 100 | 25 | - | 5 | 5 | 19.28 |
| Mucuma beans [89] | NaOH | 100 | 25 | - | 5 | 7,8 | 19.93 |
| Orange tree sawdust [90] | NaOH | 40–100 | 20 | 3 | 1 | 6 | 78.74 |
| Parthenium hysterophorus [91] | H3PO4 | 50–250 | 26 | 1.5 | 4 | 7 | 88.49 |
| Parthenium hysterophorus [91] | H2SO4 | 50–250 | 26 | 1.5 | 4 | 7 | 39.68 |
| Seed husk of Timbaúva [92] | - | 10–50 | 25 | - | 20 | - | 3.62 |
| Sugarcane Bagasse [93] | - | 10–50 | 30 | 1.5 | 1.5 | 6 | 1.83 |
| Waste fruits of Rapanea ferrugínea [8] | H2SO4 | 20–120 | 25 | 2 | 1.2 | 7 | 33.00 |
| AC- waste of sunflower oil [94] | H2SO4 | 0–250 | 25 | 24 | 2 | 6 | 16.43 |
| AC- Coconut fiber [95] | ZnCl2 | 60–100 | 30 | 1.6 | 5 | 8 | 15.49 |
| AC- Apricot stones [96] | H3PO4 + HNO3 | 5–100 | 25 | 2 | 0.1 | 5 | 36.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, E.O.; Valério Filho, A.; de Araujo, E.B.; Andrade, T.D.; dos Santos, M.C.; Zottis, R.; da Rosa, G.S.; de Almeida, A.R.F. Application of Lolium multiflorum as an Efficient Raw Material in the Production of Adsorbent for Removal of Methylene Blue. C 2023, 9, 44. https://doi.org/10.3390/c9020044
da Silva EO, Valério Filho A, de Araujo EB, Andrade TD, dos Santos MC, Zottis R, da Rosa GS, de Almeida ARF. Application of Lolium multiflorum as an Efficient Raw Material in the Production of Adsorbent for Removal of Methylene Blue. C. 2023; 9(2):44. https://doi.org/10.3390/c9020044
Chicago/Turabian Styleda Silva, Elenara Oliveira, Alaor Valério Filho, Emanuelle Butato de Araujo, Taís Douglas Andrade, Maele Costa dos Santos, Ricardo Zottis, Gabriela Silveira da Rosa, and André Ricardo Felkl de Almeida. 2023. "Application of Lolium multiflorum as an Efficient Raw Material in the Production of Adsorbent for Removal of Methylene Blue" C 9, no. 2: 44. https://doi.org/10.3390/c9020044
APA Styleda Silva, E. O., Valério Filho, A., de Araujo, E. B., Andrade, T. D., dos Santos, M. C., Zottis, R., da Rosa, G. S., & de Almeida, A. R. F. (2023). Application of Lolium multiflorum as an Efficient Raw Material in the Production of Adsorbent for Removal of Methylene Blue. C, 9(2), 44. https://doi.org/10.3390/c9020044