Graphene Oxide: A Comparison of Reduction Methods
Abstract
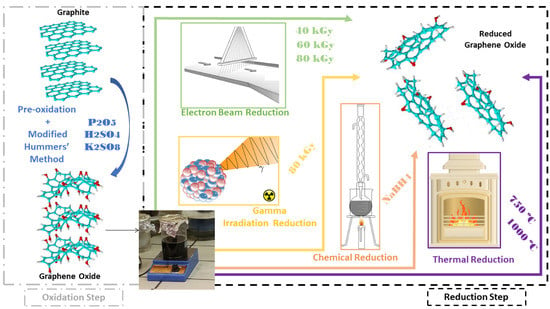
:1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene Oxide
2.2. Preparation of Reduced Graphene Oxide by Chemical Reduction
2.3. Preparation of Reduced Graphene Oxide via Thermal Reduction
2.4. Preparation of Reduced Graphene Oxide by Gamma Radiation Reduction
2.5. Preparation of Reduced Graphene Oxide by Electron Beam Reduction
2.6. Characterization
3. Results
3.1. Raman Spectroscopy
3.2. X-ray Diffraction (XRD)
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Energy-Dispersive Spectrometry (EDS)
3.5. Scanning Electron Microscopy (SEM)
3.6. Atomic Force Microscopy (AFM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Ng, Y.H.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202218016. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Sriram, B.; Muthukutty, B.; Zheng, L.; Wang, S.-F.; Choe, H.; Kwon, K. Layered metal chalcogenide of SnSe nanosheets integrated with 2D-hexagonal boron nitride for accurate and low-level detection of nitrofurazone. Chem. Eng. J. 2023, 455, 140521. [Google Scholar] [CrossRef]
- Ashraf, M.; Hemasiri, N.H.; Kazim, S.; Ullah, N.; Khan, M.; Ganiyu, S.A.; Alhooshani, K.R.; Tahir, M.N.; Ahmad, S. Interface engineering of a hole-transport layer/perovskite with low-band-gap 2D-carbon nitrides for solar cell fabrication. Sustain. Energy Fuels 2023, 7, 763–768. [Google Scholar] [CrossRef]
- Liu, L.; Feng, J.; Xue, Y.; Chevali, V.; Zhang, Y.; Shi, Y.; Tang, L.; Song, P. 2D MXenes for Fire Retardancy and Fire-Warning Applications: Promises and Prospects. Adv. Funct. Mater. 2023, 33, 2212124. [Google Scholar] [CrossRef]
- Geng, D.; Yang, H.Y. Recent Advances in Growth of Novel 2D Materials: Beyond Graphene and Transition Metal Dichalcogenides. Adv. Mater. 2018, 30, e1800865. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Wang, C. Liquid-Based Exfoliation of Black Phosphorus into Phosphorene and Its Application for Energy Storage Devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Saedi, M.; Mohseni, S.M.; Groot, I.M. Thermodynamic analysis of graphene CVD grown on liquid metal: Growth on liquid metallic gallium or solid gallium oxide skin? Mater. Chem. Phys. 2022, 275, 125203. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Tambe, P. Synthesis and characterization of acid treated reduced graphene oxide. Mater. Today Proc. 2021, 49, 1294–1297. [Google Scholar] [CrossRef]
- Ratova, D.-M.V.; Mikheev, I.V.; Chermashentsev, G.R.; Maslakov, K.I.; Kottsov, S.Y.; Stolbov, D.N.; Maksimov, S.V.; Sozarukova, M.M.; Proskurnina, E.V.; Proskurnin, M.A. Green and Sustainable Ultrasound-Assisted Anodic Electrochemical Preparation of Graphene Oxide Dispersions and Their Antioxidant Properties. Molecules 2023, 28, 3238. [Google Scholar] [CrossRef] [PubMed]
- Suranshe, S.S.; Patil, A. Strategically improving electrical conductivity of reduced graphene oxide through a series of reduction processes. Mater. Lett. 2023, 333, 133648. [Google Scholar] [CrossRef]
- Khakpour, I.; Baboukani, A.R.; Allagui, A.; Wang, C. Bipolar Exfoliation and In Situ Deposition of High-Quality Graphene for Supercapacitor Application. ACS Appl. Energy Mater. 2019, 2, 4813–4820. [Google Scholar] [CrossRef]
- McDonald, M.P.; Morozov, Y.; Hodak, J.H.; Kuno, M. Spectroscopy and Microscopy of Graphene Oxide and Reduced Graphene Oxide. In Graphene Oxide; Gao, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 29–60. [Google Scholar] [CrossRef]
- Powell, C.; Beall, G.W. Graphene oxide and graphene from low grade coal: Synthesis, characterization and applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 362–366. [Google Scholar] [CrossRef]
- Collins Brodie, B. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tienne, L.G.P.; Candido, L.D.S.; Cruz, B.D.S.M.D.; Gondim, F.F.; Ribeiro, M.P.; Simão, R.A.; Marques, M.D.F.V.; Monteiro, S.N. Reduced graphene oxide synthesized by a new modified Hummer’s method for enhancing thermal and crystallinity properties of Poly(vinylidene fluoride). J. Mater. Res. Technol. 2022, 18, 4871–4893. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Tiwari, S.; Purkait, M.K. A comprehensive review on graphene oxide-based nanocarriers: Synthesis, functionalization and biomedical applications. Flatchem 2023, 38, 100484. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, S.; Liu, L.; Liu, X. High-quality preparation of graphene oxide via the Hummers’ method: Understanding the roles of the intercalator, oxidant, and graphite particle size. Ceram. Int. 2020, 46, 2392–2402. [Google Scholar] [CrossRef]
- Aixart, J.; Díaz, F.; Llorca, J.; Rosell-Llompart, J. Increasing reaction time in Hummers’ method towards well exfoliated graphene oxide of low oxidation degree. Ceram. Int. 2021, 47, 22130–22137. [Google Scholar] [CrossRef]
- Ucar, N.; Yuksek, I.O.; Olmez, M.; Can, E.; Onen, H. The effect of oxidation process on graphene oxide fiber properties. Mater. Sci. 2019, 37, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Drewniak, S.; Pustelny, T.; Muzyka, R.; Stolarczyk, A.; Konieczny, G. Investigations of selected physical properties of graphite oxide and thermally exfoliated/reduced graphene oxide in the aspect of their applications in photonic gas sensors. Photon. Lett. Pol. 2015, 7, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.; Saleem, M.; Ullah, S.; Saeed, N.; Afridi, A.; Khan, M.; Arif, M. Modified and improved Hummer’s synthesis of graphene oxide for capacitors applications. Mod. Electron. Mater. 2017, 3, 110–116. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Li, F.; Lei, W.; Gao, Y.; Wu, Y.; Zhang, L.; Wang, Z.L. Nanoparticle chemically end-linking elastomer network with super-low hysteresis loss for fuel-saving automobile. Nano Energy 2016, 28, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Wang, Y.; Chen, L.; Huang, L.; Chen, Y. Control of the oxidation level of graphene oxide for high efficiency polymer solar cells. RSC Adv. 2015, 5, 49182–49187. [Google Scholar] [CrossRef]
- Nagaoka, D.A.; Grasseschi, D.; Domingues, S.H. Can reduced graphene oxide look like few-layer pristine graphene? Diam. Relat. Mater. 2021, 120, 108616. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Effect of oxidation degree on the thermal properties of graphene oxide. J. Mater. Res. Technol. 2020, 9, 13740–13748. [Google Scholar] [CrossRef]
- Kavitha, C. A review on reduced Graphene oxide hybrid nano composites and their prominent applications. Mater. Today Proc. 2021, 49, 811–816. [Google Scholar] [CrossRef]
- Hada, M.; Miyata, K.; Ohmura, S.; Arashida, Y.; Ichiyanagi, K.; Katayama, I.; Suzuki, T.; Chen, W.; Mizote, S.; Sawa, T.; et al. Selective Reduction Mechanism of Graphene Oxide Driven by the Photon Mode versus the Thermal Mode. ACS Nano 2019, 13, 10103–10112. [Google Scholar] [CrossRef]
- Feng, J.; Ye, Y.; Xiao, M.; Wu, G.; Ke, Y. Synthetic routes of the reduced graphene oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Sieradzka, M.; Ślusarczyk, C.; Fryczkowski, R.; Janicki, J. Insight into the effect of graphite grain sizes on the morphology, structure and electrical properties of reduced graphene oxide. J. Mater. Res. Technol. 2020, 9, 7059–7067. [Google Scholar] [CrossRef]
- Koreshkova, A.N.; Gupta, V.; Peristyy, A.; Nesterenko, P.N.; Rodemann, T.; Paull, B. Ion chromatographic determination of hydrazine in excess ammonia for monitoring graphene oxide reduction reaction. Talanta 2019, 2005, 120081. [Google Scholar] [CrossRef]
- Hu, J.; Kong, G.; Zhu, Y.; Che, C. Ultrafast room-temperature reduction of graphene oxide by sodium borohydride, sodium molybdate and hydrochloric acid. Chin. Chem. Lett. 2021, 32, 543–547. [Google Scholar] [CrossRef]
- Poorali, M.-S.; Bagheri-Mohagheghi, M.-M. Comparison of chemical and physical reduction methods to prepare layered graphene by graphene oxide: Optimization of the structural properties and tuning of energy band gap. J. Mater. Sci. Mater. Electron. 2016, 27, 260–271. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Wang, Z.; Xie, S.; Zhang, Y.; Shen, Y.; Yu, M.; Deng, B.; Huang, Q.; Fan, C.; et al. Radiation induced reduction: An effective and clean route to synthesize functionalized graphene. J. Mater. Chem. 2012, 22, 7775–7781. [Google Scholar] [CrossRef]
- Ershov, B.G. Kinetics, mechanism and intermediates of some radiation-induced reactions in aqueous solutions. Russ. Chem. Rev. 2004, 73, 101–113. [Google Scholar] [CrossRef]
- Santana, J.G.; Akbulut, M.; Temperini, M.L.; Rangari, V.K.; Güven, O.; Moura, E. Synergistic effect of e-beam irradiation and graphene oxide incorporation on thermal, mechanical, and barrier properties of poly (ethylene-co-vinyl alcohol) film. Radiat. Phys. Chem. 2022, 199, 110343. [Google Scholar] [CrossRef]
- Changotra, R.; Guin, J.P.; Varshney, L.; Dhir, A. Assessment of reaction intermediates of gamma radiation-induced degradation of ofloxacin in aqueous solution. Chemosphere 2018, 208, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Soroka, I.; Chae, N.; Jonsson, M. On the mechanism of γ-radiation-induced corrosion of copper in water. Corros. Sci. 2021, 182, 109279. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Li, D.-Y.; Yi, R.-B.; Mo, J.-W.; Wu, M.-H.; Xu, G. Controllable reduction of graphene oxide by electron-beam irradiation. RSC Adv. 2019, 9, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Jung, C.-H.; Oh, M.-S.; Hwang, I.-T.; Shin, K.; Hwang, J.; Park, S.-H.; Choi, J.-H. Rapid, facile, and eco-friendly reduction of graphene oxide by electron beam irradiation in an alcohol–water solution. Mater. Lett. 2014, 126, 151–153. [Google Scholar] [CrossRef]
- Atta, M.M.; Maksoud, M.I.A.A.; Sallam, O.I.; Awed, A.S. Gamma irradiation synthesis of wearable supercapacitor based on reduced graphene oxide/cotton yarn electrode. J. Mater. Sci. Mater. Electron. 2021, 32, 3688–3698. [Google Scholar] [CrossRef]
- Jacovone, R.M.S.; Tominaga, F.K.; Brandão, O.A.B.; Garcia, R.H.L.; Sakata, S.K. Synthesis of Reduced Graphene Oxide by Gamma Irradiation. In Proceedings of the International Nuclear Atlantic Conference, Santos, Brazil, 21–25 October 2019; Associação Brasileira De Energia Nuclear: Santos, SP, Brazil, 2019; pp. 2398–2405. [Google Scholar]
- Ansón-Casaos, A.; Puértolas, J.; Pascual, F.; Hernández-Ferrer, J.; Castell, P.; Benito, A.; Maser, W.; Martínez, M. The effect of gamma-irradiation on few-layered graphene materials. Appl. Surf. Sci. 2014, 301, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Somessari, S.L.; Somessari, E.S.R.; Da Silveira, C.G.; Calvo, W.A.P. Analysis of the Power System from an Electron Beam Accelerator and the Correlation with the Theoretical Dosimetry for Radiation Processing. J. Phys. Sci. Appl. 2015, 5, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Soares, J.; Oliveros, M.; Garin, C.; David, M.; Martins, L.; Almeida, C.; Martins-Ferreira, E.; Takai, K.; Enoki, T.; Magalhães-Paniago, R.; et al. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon 2015, 95, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, A.; Dhawan, S.K.; Singh, V. Determination of crystallite size, number of graphene layers and defect density of graphene oxide (GO) and reduced graphene oxide (RGO). AIP Conf. Proc. 2019, 2115, 030106. [Google Scholar] [CrossRef]
- Rantitsch, G.; Lämmerer, W.; Fisslthaler, E.; Mitsche, S.; Kaltenböck, H. On the discrimination of semi-graphite and graphite by Raman spectroscopy. Int. J. Coal Geol. 2016, 159, 48–56. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roscher, S.; Hoffmann, R.; Ambacher, O. Determination of the graphene–graphite ratio of graphene powder by Raman 2D band symmetry analysis. Anal. Methods 2019, 11, 1180–1191. [Google Scholar] [CrossRef] [Green Version]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman Spectroscopy of Graphene and Related Materials. New Developments in Photon and Materials Research. 1. 2013. Available online: https://www.physics.purdue.edu/quantum/files/Raman_Spectroscopy_of_Graphene_NOVA_Childres.pdf (accessed on 11 July 2023).
- Selhorst, R.; Susner, M.A.; Muzzio, R.; Kao, I.-H.; Carpena-Núñez, J.; Islam, A.E.; Katoch, J.; Maruyama, B.; Rao, R. Electron-beam chemistry in graphene—Effect of environmental SEM parameters on patterning and defect engineering. Vacuum 2023, 207, 111686. [Google Scholar] [CrossRef]
- Caicedo, F.M.C.; López, E.V.; Agarwal, A.; Drozd, V.; Durygin, A.; Hernandez, A.F.; Wang, C. Synthesis of graphene oxide from graphite by ball milling. Diam. Relat. Mater. 2020, 109, 108064. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Fujimoto, H. Theoretical X-ray scattering intensity of carbons with turbostratic stacking and AB stacking structures. Carbon 2003, 41, 1585–1592. [Google Scholar] [CrossRef]
- Popova, A. Crystallographic analysis of graphite by X-Ray diffraction. Coke Chem. 2017, 60, 361–365. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Pavoski, G.; Maraschin, T.; Fim, F.D.C.; Balzaretti, N.M.; Galland, G.B.; Moura, C.S.; Basso, N.R.D.S. Few Layer Reduced Graphene Oxide: Evaluation of the Best Experimental Conditions for Easy Production. Mater. Res. 2017, 20, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.C.; Kheireddine, S.; Dandach, A.; Eternot, M.; Vu, T.T.H.; Essayem, N. Acid Properties of GO and Reduced GO as Determined by Microcalorimetry, FTIR, and Kinetics of Cellulose Hydrolysis-Hydrogenolysis. Catalysts 2020, 10, 1393. [Google Scholar] [CrossRef]
- Perera, D.; Abeywickrama, A.; Zen, F.; Colavita, P.E.; Jayasundara, D.R. Evolution of oxygen functionalities in graphene oxide and its impact on structure and exfoliation: An oxidation time based study. Mater. Chem. Phys. 2018, 220, 417–425. [Google Scholar] [CrossRef]
- Chuah, R.; Gopinath, S.C.B.; Anbu, P.; Salimi, M.N.; Yaakub, A.R.W.; Lakshmipriya, T. Synthesis and characterization of reduced graphene oxide using the aqueous extract of Eclipta prostrata. 3 Biotech 2020, 10, 364. [Google Scholar] [CrossRef]
- Romero, A.; Lavin-Lopez, M.; Sanchez-Silva, L.; Valverde, J.; Paton-Carrero, A. Comparative study of different scalable routes to synthesize graphene oxide and reduced graphene oxide. Mater. Chem. Phys. 2018, 203, 284–292. [Google Scholar] [CrossRef] [Green Version]









| Nomenclature | Samples Description |
|---|---|
| GRF | Graphite |
| GO | Graphene oxide |
| rGOq | Reduced graphene oxide by chemical reduction |
| rGOt750 | Reduced graphene oxide by thermal reduction at 750 °C |
| rGOt1000 | Reduced graphene oxide by thermal reduction at 1000 °C |
| rGOe40 | Reduced graphene oxide by electron beam reduction at 40 kGy |
| rGOe60 | Reduced graphene oxide by electron beam reduction at 60 kGy |
| rGOe80 | Reduced graphene oxide by electron beam reduction at 80 kGy |
| rGOg | Reduced graphene oxide by gamma radiation |
| D Band/FWHM (cm−1) | G Band/FWHM (cm−1) | 2D Band (cm−1) | La (nm) | ID/IG | |
|---|---|---|---|---|---|
| GRF | 1366/49.9 | 1593/22.8 | 2730 | 104.0 | 0.18 |
| GO | 1370/207.6 | 1608/170.1 | 2668 | 18.3 | 1.05 |
| rGOq | 1341/99.8 | 1588/89.5 | 2679 | 15.9 | 1.21 |
| rGOe80 | 1343/75.1 | 1572/71.8 | 2665 | 15.6 | 1.23 |
| rGOe60 | 1343/81.5 | 1574/77.7 | 2679 | 16.9 | 1.14 |
| rGOe40 | 1343/92.7 | 1578/82.5 | 2669 | 17.9 | 1.07 |
| rGOg | 1341/83.6 | 1576/74.5 | 2679 | 15.9 | 1.21 |
| rGOt750 | 1344/193.9 | 1585/174.3 | 2657 | 19.3 | 1.00 |
| rGOt1000 | 1344/170.6 | 1585/111.5 | 2647 | 18.7 | 1.03 |
| 2θ (°) | d (Å) | |
|---|---|---|
| GRF | 26.3 | 3.4 |
| GO | 42.3/10.1 | 2.1/8.7 |
| rGOq | 43.1/25.3/10.9 | 2.1/3.5/8.1 |
| rGOg | 43/22.2/11.3 | 2.1/4.0/7.8 |
| rGOt750 | 43.8/24.7 | 2.1/3.6 |
| rGOe80 | 42.8/22.2/11.1 | 2.1/4.0/7.9 |
| rGOe60 | 42.7/22.1/10.7 | 2.1/4.0/8.2 |
| rGOe40 | 42.7/22.6/10.5 | 2.1/3.9/8.4 |
| C (%) | O (%) | Na (%) | C/O | |
|---|---|---|---|---|
| GO | 60.8 | 39.2 | 0 | 1.6 |
| rGOq | 70.3 | 23.7 | 6.0 | 3.0 |
| rGOe80 | 84.4 | 15.6 | 0 | 5.4 |
| rGOe60 | 74.4 | 25.6 | 0 | 2.9 |
| rGOe40 | 71.7 | 28.3 | 0 | 2.5 |
| rGOg | 80.3 | 19.7 | 0 | 4.1 |
| rGOt750 | 79.1 | 20.9 | 0 | 3.8 |
| rGOt1000 | 86.1 | 13.9 | 0 | 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros, N.G.; Gonzaga Neto, A.C.; Vaccioli, K.B.; Angulo, H.R.V.; de Andrade e Silva, L.G.; Toffoli, S.M.; Valera, T.S. Graphene Oxide: A Comparison of Reduction Methods. C 2023, 9, 73. https://doi.org/10.3390/c9030073
de Barros NG, Gonzaga Neto AC, Vaccioli KB, Angulo HRV, de Andrade e Silva LG, Toffoli SM, Valera TS. Graphene Oxide: A Comparison of Reduction Methods. C. 2023; 9(3):73. https://doi.org/10.3390/c9030073
Chicago/Turabian Stylede Barros, Natália Garrote, Abel Cardoso Gonzaga Neto, Kleber Bitencourt Vaccioli, Hugo Rafael Vallejo Angulo, Leonardo Gondim de Andrade e Silva, Samuel Marcio Toffoli, and Ticiane Sanches Valera. 2023. "Graphene Oxide: A Comparison of Reduction Methods" C 9, no. 3: 73. https://doi.org/10.3390/c9030073
APA Stylede Barros, N. G., Gonzaga Neto, A. C., Vaccioli, K. B., Angulo, H. R. V., de Andrade e Silva, L. G., Toffoli, S. M., & Valera, T. S. (2023). Graphene Oxide: A Comparison of Reduction Methods. C, 9(3), 73. https://doi.org/10.3390/c9030073