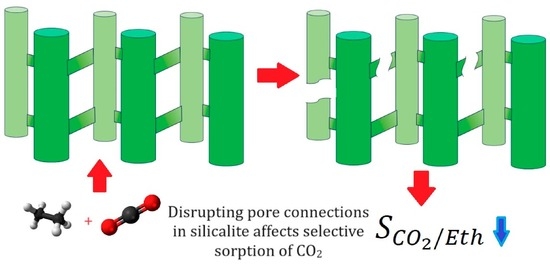
Ethane-CO2 Mixture Adsorption in Silicalite: Influence of Tortuosity and Connectivity of Pores on Selectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Models
2.2. Force Fields
2.3. Simulations
3. Results
3.1. Adsorption Isotherms
3.2. Adsorption Selectivity and Its Reversal
3.3. Comparison of Adsorption as Single Species and in Mixture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Kerry, F.G. Industrial Gas Handbook: Gas Separation and Purification; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Yang, R.T. Gas Separation by Adsorption Processes; World Scientific: Singapore, 1997; Volume 1. [Google Scholar]
- Ruthven, D.M. Molecular sieve separations. Chem. Ing. Tech. 2011, 83, 44–52. [Google Scholar] [CrossRef]
- Wang, H.; Luo, D.; Velasco, E.; Yu, L.; Li, J. Separation of alkane and alkene mixtures by metal–organic frameworks. J. Mater. Chem. A 2021, 9, 20874–20896. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D.R. Effects of Pore Connectivity on the Sorption of Fluids in Nanoporous Material: Ethane and CO2 Sorption in Silicalite. ChemEngineering 2021, 5, 55. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D.R. Effects of pore connectivity and tortuosity on the dynamics of fluids confined in sub-nanometer pores. Phys. Chem. Chem. Phys. 2022, 24, 11836–11847. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Hodnebrog, Ø.; Dalsøren, S.B.; Myhre, G. Lifetimes, direct and indirect radiative forcing, and global warming potentials of ethane (C2H6), propane (C3H8), and butane (C4H10). Atmos. Sci. Lett. 2018, 19, e804. [Google Scholar] [CrossRef]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.; Jana, S. Advances in porous adsorbents for CO2 capture and storage. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen: London, UK, 2018; pp. 165–183. [Google Scholar]
- Lemmon, E.W. Thermophysical properties of fluid systems. In NIST Chemistry WebBook; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
- He, Y.; Seaton, N.A. Experimental and computer simulation studies of the adsorption of ethane, carbon dioxide, and their binary mixtures in MCM-41. Langmuir 2003, 19, 10132–10138. [Google Scholar] [CrossRef]
- Gautam, S.; Liu, T.; Cole, D. Sorption, structure and dynamics of CO2 and ethane in silicalite at high pressure: A combined Monte Carlo and molecular dynamics simulation study. Molecules 2018, 24, 99. [Google Scholar] [CrossRef]
- Aguilar-Armenta, G.; Romero-Pérez, Á. Adsorption of C2H4, C2H6 and CO2 on cation-exchanged clinoptilolite. Adsorpt. Sci. Technol. 2009, 27, 523–536. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D.R. Effects of inter-crystalline space on the adsorption of ethane and CO2 in silicalite: Implications for enhanced adsorption. Phys. Chem. Chem. Phys. 2020, 22, 13951–13957. [Google Scholar] [CrossRef]
- Anson, A.; Lin, C.C.; Kuznicki, S.M.; Sawada, J.A. Adsorption of carbon dioxide, ethane, and methane on titanosilicate type molecular sieves. Chem. Eng. Sci. 2009, 64, 3683–3687. [Google Scholar] [CrossRef]
- Chathoth, S.M.; He, L.; Mamontov, E.; Melnichenko, Y.B. Effect of carbon dioxide and nitrogen on the diffusivity of methane confined in nano-porous carbon aerogel. Microporous Mesoporous Mater. 2012, 148, 101–106. [Google Scholar] [CrossRef]
- Salles, F.; Jobic, H.; Devic, T.; Guillerm, V.; Serre, C.; Koza, M.M.; Ferey, G.; Maurin, G. Diffusion of binary CO2/CH4 mixtures in the MIL-47 (V) and MIL-53 (Cr) metal–organic framework type solids: A combination of neutron scattering measurements and molecular dynamics simulations. J. Phys. Chem. C 2013, 117, 11275–11284. [Google Scholar] [CrossRef]
- Elola, M.D.; Rodriguez, J. Preferential adsorption in ethane/carbon dioxide fluid mixtures confined within silica nanopores. J. Phys. Chem. C 2019, 123, 30937–30948. [Google Scholar] [CrossRef]
- Dhiman, I.; Bhowmik, D.; Shrestha, U.R.; Cole, D.R.; Gautam, S. Effect of molecular shape on rotation under severe confinement. Chem. Eng. Sci. 2018, 180, 33–41. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Van Koningsveld, H.; Van Bekkum, H.; Jansen, J.C. On the location and disorder of the tetrapropylammonium (TPA) ion in zeolite ZSM-5 with improved framework accuracy. Acta Crystallogr. Sect. B Struct. Sci. 1987, 43, 127–132. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Transferable potentials for phase equilibria. 1. United-atom description of n-alkanes. J. Phys. Chem. B 1998, 102, 2569–2577. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Purton, J.A.; Crabtree, J.C.; Parker, S.C. DL_MONTE: A general purpose program for parallel Monte Carlo simulation. Mol. Simul. 2013, 39, 1240–1252. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Pham, T.D.; Lobo, R.F. Adsorption equilibria of CO2 and small hydrocarbons in AEI-, CHA-, STT-, and RRO-type siliceous zeolites. Microporous Mesoporous Mater. 2016, 236, 100–108. [Google Scholar] [CrossRef]
- Sun, M.S.; Shah, D.B.; Xu, H.H.; Talu, O. Adsorption Equilibria of C1 to C4 Alkanes, CO2, and SF6 on Silicalite. J. Phys. Chem. B 1998, 102, 1466–1473. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mayadevi, S. Adsorption of methane, ethane, ethylene, and carbon dioxide on silicalite-l. Zeolites 1996, 17, 501–507. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Li, Y.; Zhang, J.; Li, M.; Li, Z.; Guo, Z.; Li, J. Shale pore connectivity and influencing factors based on spontaneous imbibition combined with a nuclear magnetic resonance experiment. Mar. Pet. Geol. 2021, 132, 105239. [Google Scholar] [CrossRef]
- Chandra, D.; Vishal, V.; Bahadur, J.; Sen, D. A novel approach to identify accessible and inaccessible pores in gas shales using combined low-pressure sorption and SAXS/SANS analysis. Int. J. Coal Geol. 2020, 228, 103556. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Hunt, A.G.; Ewing, R.P.; Sahimi, M. Tortuosity in porous media: A critical review. Soil Sci. Soc. Am. J. 2013, 77, 1461–1477. [Google Scholar] [CrossRef]
- Fu, J.; Thomas, H.R.; Li, C. Tortuosity of porous media: Image analysis and physical simulation. Earth-Sci. Rev. 2021, 212, 103439. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C.; Lang, R.; Kotz, D.; Campbell, J. Gnuplot 4.6. 6: An Interactive Plotting Program. 2014. Available online: http://gnuplot.info (accessed on 4 October 2023).








| System Name | Open Straight Channels (% of Total) | Open Sinusoidal Channels (% of Total) | Number of Pore Connections |
|---|---|---|---|
| S4Z4 | 100 | 100 | 48 |
| S4Z3 | 100 | 75 | 36 |
| S4Z2 | 100 | 50 | 24 |
| S4Z1 | 100 | 25 | 12 |
| S4Z0 | 100 | 0 | 0 |
| S3Z1 | 75 | 25 | 9 |
| S2Z2 | 50 | 50 | 12 |
| S1Z3 | 25 | 75 | 9 |
| S0Z4 | 0 | 100 | 0 |
| S1Z4 | 25 | 100 | 12 |
| S2Z4 | 50 | 100 | 24 |
| S3Z4 | 75 | 100 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, S.; Cole, D. Ethane-CO2 Mixture Adsorption in Silicalite: Influence of Tortuosity and Connectivity of Pores on Selectivity. C 2023, 9, 116. https://doi.org/10.3390/c9040116
Gautam S, Cole D. Ethane-CO2 Mixture Adsorption in Silicalite: Influence of Tortuosity and Connectivity of Pores on Selectivity. C. 2023; 9(4):116. https://doi.org/10.3390/c9040116
Chicago/Turabian StyleGautam, Siddharth, and David Cole. 2023. "Ethane-CO2 Mixture Adsorption in Silicalite: Influence of Tortuosity and Connectivity of Pores on Selectivity" C 9, no. 4: 116. https://doi.org/10.3390/c9040116
APA StyleGautam, S., & Cole, D. (2023). Ethane-CO2 Mixture Adsorption in Silicalite: Influence of Tortuosity and Connectivity of Pores on Selectivity. C, 9(4), 116. https://doi.org/10.3390/c9040116