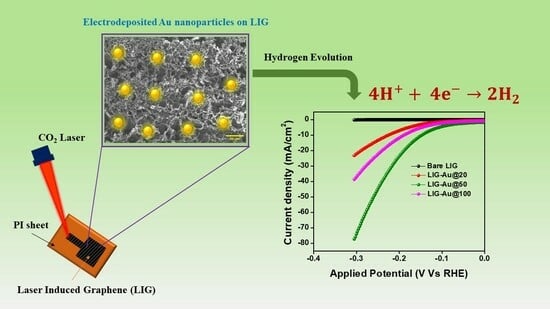
Fabrication of Gold Nanoparticles Embedded Laser-Induced Graphene (LIG) Electrode for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
Fabrication of Laser-Induced Graphene Film-Based Gold Nanocomposites
2.2. Characterization Details
2.3. Electrochemical Measurement Details
3. Results
3.1. XRD Analysis
3.2. Contact Angle Measurements
3.3. SEM Analysis
3.4. Electrochemical Performance
4. Discussion
- (1)
- The utilization of nickel foam as a substrate results in the establishment of robust interfacial contact with the active materials. The objective is to decrease the interfacial resistance and enhance the kinetics of charge transfer [50].
- (2)
- The measurement of the contact angle can yield valuable insights into the availability and accessibility of the active sites present on the surface of the electrode. A surface exhibiting a reduced contact angle is inclined to possess a larger proportion of its surface area that is accessible to the electrolyte [51]. An enlarged electrochemically active surface area (ECSA) often leads to enhanced catalytic activity in the hydrogen evolution reaction (HER). The least water contact angle of LIG-Au@50 among others supports its better catalytic activity.
- (3)
- The interplay between gold nanoparticles and laser-induced graphene has the potential to provide synergistic outcomes that augment the catalytic efficacy of both substances at moderate level of gold concentration (50 cycles). An increased quantity of gold on the (LIG) surface, specifically after 100 cycles, induces significant agglomeration, hence diminishing the catalytic activity. Conversely, a lower quantity of gold on the LIG surface, particularly after 20 cycles, exhibits an inadequate number of active sites for catalytic reactions.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Cheng, S.Y.; Li, J.B.; Huang, Y.F. Mitigating Environmental Pollution and Impacts from Fossil Fuels: The Role of Alternative Fuels. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 1069–1080. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Sangmesh, B.; Karuppasamy, K.S.K. Renewable and Sustainable Clean Energy Development and Impact on Social, Economic, and Environmental Health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Biomass Energy and the Environmental Impacts Associated with Its Production and Utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent Progress of Transition Metal Nitrides for Efficient Electrocatalytic Water Splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Sahin, N.E.; Pech-Rodríguez, W.J.; Meléndez-González, P.C.; Lopez Hernández, J.; Rocha-Rangel, E. Water Splitting as an Alternative for Electrochemical Hydrogen and Oxygen Generation: Current Status, Trends, and Challenges. Energies 2023, 16, 5078. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Cai, P.; Wen, Z. An Electrochemically Neutralized Energy-Assisted Low-Cost Acid-Alkaline Electrolyzer for Energy-Saving Electrolysis Hydrogen Generation. J. Mater. Chem. A 2018, 6, 4948–4954. [Google Scholar] [CrossRef]
- Yu, X.; Araujo, R.B.; Qiu, Z.; Campos dos Santos, E.; Anil, A.; Cornell, A.; Pettersson, L.G.M.; Johnsson, M. Hydrogen Evolution Linked to Selective Oxidation of Glycerol over CoMoO4—A Theoretically Predicted Catalyst. Adv. Energy Mater. 2022, 12, 2103750. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An Overview on Pd-Based Electrocatalysts for the Hydrogen Evolution Reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Non-Noble Metal Electrocatalysts for the Hydrogen Evolution Reaction in Water Electrolysis. Electrochem. Energy Rev. 2021, 4, 473–507. [Google Scholar] [CrossRef]
- Ibn Shamsah, S.M. Earth-Abundant Electrocatalysts for Water Splitting: Current and Future Directions. Catalysts 2021, 11, 429. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, L.; Zhou, H.; Li, D.; Tang, D.; Yu, F. Innovative Strategies in Design of Transition Metal-Based Catalysts for Large-Current-Density Alkaline Water/Seawater Electrolysis. Mater. Today Phys. 2022, 26, 100727. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Sun, C.; Xi, P.; Peng, S.; Gao, D.; Xue, D. Accelerated Hydrogen Evolution Reaction in CoS2 by Transition-Metal Doping. ACS Energy Lett. 2018, 3, 779–786. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Dou, Y.; Du, Z.; Shao, M. The Role of Transition Metal and Nitrogen in Metal–N–C Composites for Hydrogen Evolution Reaction at Universal PHs. J. Phys. Chem. C 2016, 120, 29047–29053. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Chen, S.; Vasileff, A.; Li, L.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S.-Z. Heteroatom-Doped Transition Metal Electrocatalysts for Hydrogen Evolution Reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Mott, N.F. The Basis of the Electron Theory of Metals, with Special Reference to the Transition Metals. Proc. Phys. Soc. Sect. A 1949, 62, 416. [Google Scholar] [CrossRef]
- Song, J.; Zhu, C.; Xu, B.Z.; Fu, S.; Engelhard, M.H.; Ye, R.; Du, D.; Beckman, S.P.; Lin, Y. Bimetallic Cobalt-Based Phosphide Zeolitic Imidazolate Framework: CoPx Phase-Dependent Electrical Conductivity and Hydrogen Atom Adsorption Energy for Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1601555. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.-R.; Xiao, Y.-Y.; Yin, Y.-C.; Huang, W.-M.; Huang, Z.-C.; Zheng, Y.-Z.; Mu, F.-Y.; Huang, R.; Shi, G.-Y.; et al. Electronic Metal–Support Interaction Modulates Single-Atom Platinum Catalysis for Hydrogen Evolution Reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Selvaraj, V.; Alagar, M. Pt and Pt–Ru Nanoparticles Decorated Polypyrrole/Multiwalled Carbon Nanotubes and Their Catalytic Activity towards Methanol Oxidation. Electrochem. Commun. 2007, 9, 1145–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Liao, H.; Sun, S.; Li, S.; Ager, J.W.; Xu, Z.J. Activation Effect of Electrochemical Cycling on Gold Nanoparticles towards the Hydrogen Evolution Reaction in Sulfuric Acid. Electrochim. Acta 2016, 209, 440–447. [Google Scholar] [CrossRef]
- Qiao, H.; Li, Z.; Liu, F.; Ma, Q.; Ren, X.; Huang, Z.; Liu, H.; Deng, J.; Zhang, Y.; Liu, Y.; et al. Au Nanoparticle Modification Induces Charge-Transfer Channels to Enhance the Electrocatalytic Hydrogen Evolution Reaction of InSe Nanosheets. ACS Appl. Mater. Interfaces 2022, 14, 2908–2917. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Nguyen, M.T.T.; Le, H.V.; Nguyen, D.N.; Truong, Q.D.; Tran, P.D. Gold Nanoparticles as an Outstanding Catalyst for the Hydrogen Evolution Reaction. Chem. Commun. 2018, 54, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, D.-W.; Wang, Y.-S.; Fu, C. In Situ Growth of Different Numbers of Gold Nanoparticles on MoS2 with Enhanced Electrocatalytic Activity for Hydrogen Evolution Reaction. Chin. Phys. B 2018, 27, 68103. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.; Li, Y.; Li, M.-Y.; Zhang, H.; Zhang, J. Boosting Electrocatalytic Hydrogen Evolution by Plasmon-Driven Hot-Electron Excitation. Nanoscale 2018, 10, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Recent Development of Transition Metal Doped Carbon Materials Derived from Biomass for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2022, 47, 32436–32454. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Hui, X.; Xuan, X.; Kim, J.; Park, J.Y. A Highly Flexible and Selective Dopamine Sensor Based on Pt-Au Nanoparticle-Modified Laser-Induced Graphene. Electrochim. Acta 2019, 328, 135066. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Sha, J.; Fei, H.; Li, Y.; Tour, J.M. Efficient Water-Splitting Electrodes Based on Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2017, 9, 26840–26847. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Chen, B.; Johnson, Z.; Wilkins, A.; Sanborn, D.; Figueroa-Felix, N.; Mendivelso-Perez, D.; Smith, E.A.; Gomes, C.; Claussen, J.C. Laser-Induced Graphene Electrodes for Electrochemical Ion Sensing, Pesticide Monitoring, and Water Splitting. Anal. Bioanal. Chem. 2021, 413, 6201–6212. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef] [PubMed]
- Sneha, K.; Esterle, A.; Sharma, N.; Sahi, S. Yucca-Derived Synthesis of Gold Nanomaterial and Their Catalytic Potential. Nanoscale Res. Lett. 2014, 9, 627. [Google Scholar] [CrossRef]
- Khalil, M.; Ismail, E.; El-Magdoub, F. Biosynthesis of Au Nanoparticles Using Olive Leaf Extract. 1st Nano Updates. Arab. J. Chem. 2012, 5, 431–437. [Google Scholar] [CrossRef]
- Wilner, O.I.; Guidotti, C.; Wieckowska, A.; Gill, R.; Willner, I. Probing Kinase Activities by Electrochemistry, Contact-Angle Measurements, and Molecular-Force Interactions. Chem. A Eur. J. 2008, 14, 7774–7781. [Google Scholar] [CrossRef] [PubMed]
- Radiom, M.; Yang, C.; Chan, W.K. Characterization of Surface Tension and Contact Angle of Nanofluids. In Proceedings of the Fourth International Conference on Experimental Mechanics, Singapore, 18–20 November 2009; SPIE: Bellingham, WA, USA, 2010; Volume 7522, pp. 395–403. [Google Scholar]
- Abdelsalam, M.E.; Bartlett, P.N.; Kelf, T.; Baumberg, J. Wetting of Regularly Structured Gold Surfaces. Langmuir 2005, 21, 1753–1757. [Google Scholar] [CrossRef]
- Noor, T.; Yaqoob, L.; Iqbal, N. Recent Advances in Electrocatalysis of Oxygen Evolution Reaction Using Noble-Metal, Transition-Metal, and Carbon-Based Materials. ChemElectroChem 2021, 8, 447–483. [Google Scholar] [CrossRef]
- Raghav, J.; Deepak, D.; Sinha Roy, S.; Roy, S. Hybrid Nanostructure of Sputter Decorated Nanodots of Ag2O/AgO over Flower-like Mn–Co–Cu Ternary Metal Oxide for Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 2286–2295. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Li, J.; Huang, L.; Yao, K.; Yiu, C.K.; Liu, Y.; Wong, T.H.; Li, D.; Wu, M.; et al. Transient, Implantable, Ultrathin Biofuel Cells Enabled by Laser-Induced Graphene and Gold Nanoparticles Composite. Nano Lett. 2022, 22, 3447–3456. [Google Scholar] [CrossRef]
- Rogers, C.; Perkins, W.S.; Veber, G.; Williams, T.E.; Cloke, R.R.; Fischer, F.R. Synergistic Enhancement of Electrocatalytic CO2 Reduction with Gold Nanoparticles Embedded in Functional Graphene Nanoribbon Composite Electrodes. J. Am. Chem. Soc. 2017, 139, 4052–4061. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental Aspects and Recent Advances in Transition Metal Nitrides as Electrocatalysts for Hydrogen Evolution Reaction: A Review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Kibria, M.F.; Mridha, M.S.; Khan, A.H. Electrochemical Studies of a Nickel Electrode for the Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 1995, 20, 435–440. [Google Scholar] [CrossRef]
- Shin, S.; Jin, Z.; Kwon, D.H.; Bose, R.; Min, Y.-S. High Turnover Frequency of Hydrogen Evolution Reaction on Amorphous MoS2 Thin Film Directly Grown by Atomic Layer Deposition. Langmuir 2015, 31, 1196–1202. [Google Scholar] [CrossRef]
- Castro, E.B.; de Giz, M.J.; Gonzalez, E.R.; Vilche, J.R. An Electrochemical Impedance Study on the Kinetics and Mechanism of the Hydrogen Evolution Reaction on Nickel Molybdenite Electrodes. Electrochim. Acta 1997, 42, 951–959. [Google Scholar] [CrossRef]
- Giesbrecht, P.K.; Freund, M.S. Investigation of Hydrogen Oxidation and Evolution Reactions at Porous Pt/C Electrodes in Nafion-Based Membrane Electrode Assemblies Using Impedance Spectroscopy and Distribution of Relaxation Times Analysis. J. Phys. Chem. C 2022, 126, 132–150. [Google Scholar] [CrossRef]
- Xu, D.; Chan, K.C.; Guo, H.; Zhong, H.; Lu, L. One-Step Fabrication of a Laser-Induced Forward Transfer Graphene/Cu: XO Nanocomposite-Based Electrocatalyst to Promote Hydrogen Evolution Reaction. J. Mater. Chem. A 2021, 9, 16470–16478. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, C.; Xie, Y.; Tumlin, T.; Giri, L.; Karna, S.P.; Lin, J. Laser Induced MoS2/Carbon Hybrids for Hydrogen Evolution Reaction Catalysts. J. Mater. Chem. A 2016, 4, 6824–6830. [Google Scholar] [CrossRef]
- Khan, I.; Baig, N.; Bake, A.; Haroon, M.; Ashraf, M.; Al-Saadi, A.; Tahir, M.N.; Wooh, S. Robust Electrocatalysts Decorated Three-Dimensional Laser-Induced Graphene for Selective Alkaline OER and HER. Carbon 2023, 213, 118292. [Google Scholar] [CrossRef]
- Lyu, L.; Kang, J.; Seong, K.; Kim, C.; Lim, J.; Piao, Y. ZnNiCo Hydroxide/Graphene-Carbon Nanotube Hydrogel on Surface-Modified Ni Foam as a Battery-Type Electrode for Hybrid Supercapacitors. J. Alloys Compd. 2021, 872, 159610. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, M.J.; Kim, J.J. Impact of Surface Hydrophilicity on Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 11940–11947. [Google Scholar] [CrossRef]
- Aguas, I.; Alarcón, E.; Villa, A.L. Turpentine Valorization by Its Oxyfunctionalization to Nopol through Heterogeneous Catalysis. Heliyon 2020, 6, e03887. [Google Scholar] [CrossRef] [PubMed]
- Masoud, N.; Delannoy, L.; Calers, C.; Gallet, J.J.; Bournel, F.; de Jong, K.P.; Louis, C.; de Jongh, P.E. Silica-Supported Au–Ag Catalysts for the Selective Hydrogenation of Butadiene. ChemCatChem 2017, 9, 2418–2425. [Google Scholar] [CrossRef] [PubMed]





| S.No. | Sample Name | Overpotential (mV) | Tafel Slope (mVdec−1) | ECSA (cm2) | Mass Activity (A g−1) | TOF (s−1) |
|---|---|---|---|---|---|---|
| 1 | LIG-Au@20 | 219 | 187 | 0.30 | 2.09 | 0.00027 |
| 2 | LIG-Au@50 | 141 | 131 | 1.28 | 8.80 | 0.0091 |
| 3 | LIG-Au@100 | 183 | 154 | 0.76 | 4.29 | 0.0045 |
| Sample | Charge Transfer Resistance (ohm) | Solution Resistance (ohm) |
|---|---|---|
| LIG-Au@20 | 848.5 | 88.67 |
| LIG-Au@50 | 211.9 | 22.19 |
| LIG-Au@100 | 440.8 | 46.16 |
| S.No. | Catalyst | Electrolyte | Overpotential at 10 mA/cm2 (mV) | Reference |
|---|---|---|---|---|
| 1 | Gold nanoparticles | 0 pH H2SO4 | 200 | [23] |
| 2 | InSe Au | 0.5 M H2SO4 | 392 | [22] |
| 3 | LIG-CuO | 1M KOH | 149 | [47] |
| 4 | LIG-Mos2 | 0.5 M H2SO4 | 216 | [48] |
| 5 | Pt 3D LIG | 1 M KOH | 455 | [49] |
| 6 | LIG-Au@50 | 0.5 M H2SO4 | 141 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepak, D.; Vuruputuri, V.; Bhattacharya, G.; McLaughlin, J.A.; Roy, S.S. Fabrication of Gold Nanoparticles Embedded Laser-Induced Graphene (LIG) Electrode for Hydrogen Evolution Reaction. C 2023, 9, 118. https://doi.org/10.3390/c9040118
Deepak D, Vuruputuri V, Bhattacharya G, McLaughlin JA, Roy SS. Fabrication of Gold Nanoparticles Embedded Laser-Induced Graphene (LIG) Electrode for Hydrogen Evolution Reaction. C. 2023; 9(4):118. https://doi.org/10.3390/c9040118
Chicago/Turabian StyleDeepak, Deepak, Vennela Vuruputuri, Gourav Bhattacharya, James A. McLaughlin, and Susanta Sinha Roy. 2023. "Fabrication of Gold Nanoparticles Embedded Laser-Induced Graphene (LIG) Electrode for Hydrogen Evolution Reaction" C 9, no. 4: 118. https://doi.org/10.3390/c9040118
APA StyleDeepak, D., Vuruputuri, V., Bhattacharya, G., McLaughlin, J. A., & Roy, S. S. (2023). Fabrication of Gold Nanoparticles Embedded Laser-Induced Graphene (LIG) Electrode for Hydrogen Evolution Reaction. C, 9(4), 118. https://doi.org/10.3390/c9040118