Synthesis and Processing of Near Infrared—Activated Vitrimer Nanocomposite Films Modified with β-Hydroxyester-Functionalized Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Epoxy–Acid Linear Polymer and Vitrimer
2.2.2. Oxidation of p-MWCNTs
2.2.3. Functionalization of ox-MWCNTs
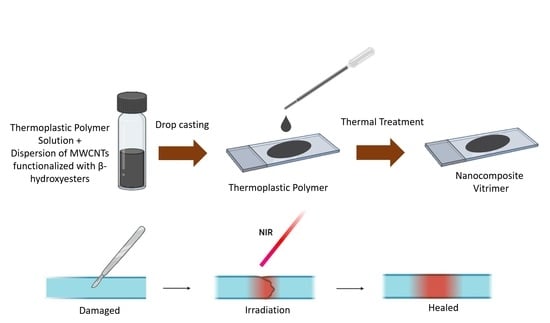
2.2.4. Nanocomposite Vitrimer Film Preparation
2.3. Characterization Techniques
2.3.1. Fourier-Transformed Infrared (FT-IR)
2.3.2. Dynamic–Mechanical Analysis (DMA) and Stress Relaxation
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Transmission Electronic Microscopy (TEM)
2.3.5. Dynamic Scanning Calorimetry (DSC)
2.3.6. Transmission Optical Microscopy (TOM)
2.3.7. Profilometry Measurements
2.3.8. Photothermal Effect Measurement
2.3.9. Raman Analysis of MWCNTs
2.3.10. Shape Memory and Welding Experiments
3. Results and Discussion
3.1. Synthesis and Characterization of the Vitrimers
3.2. Films’ Processing
3.3. Functionalization of MWCNTs
3.4. Dispersion of MWCNTs in the Vitrimer
3.5. Photothermal Effect: NIR Activation of Welding, Self-Healing, and Shape Memory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korley, L.T.J.; Epps, T.H.; Helms, B.A.; Ryan, A.J. Toward polymer upcycling—Adding value and tackling circularity. Science 2021, 73, 66–69. [Google Scholar] [CrossRef]
- Shuaib, N.A.; Sultan, A.A.M.; Ismail, S.O.; Samat, A.A.; Omar, N.W.Y.; Azmi, A.I.; Mativenga, P.T. Recycling of Composite Materials. In Advances in Machining of Composite Materials; Shyha, I., Huo, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 527–552. [Google Scholar]
- Pascault, J.-P.; Williams, R.J.J. Epoxy Polymers New Materials and Innovations; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Choudhary, A.; Prasad, E. Epoxy Resin Market by Form (Solid & Liquid), Type (DGBEA, DGBEF, Novolac, Aliphatic, Glycidylamine, and Others), End Use (Building & Construction, Transportation, General Industrial, Consumer Goods, Wind Energy, Aerospace, and Marine), and Application (Paints & Coatings, Adhesives & Sealants, Composites, Electronic Encapsulation, and Others): Global Opportunity Analysis and Industry Forecast 2021–2030. Available online: https://www.alliedmarketresearch.com/epoxy-resins-market (accessed on 17 March 2023).
- May, C.A. (Ed.) Epoxy Resins: Chemistry and Technology; M. Dekker: New York, NY, USA, 1988. [Google Scholar]
- Alabiso, W.; Schlögl, S. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Altuna, F.I.; Casado, U.; dell’Erba, I.E.; Luna, L.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers incorporating physical crosslinks produced by self-association of alkyl chains. Polym. Chem. 2020, 11, 1337–1347. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Shape memory epoxy vitrimers based on DGEBA crosslinked with dicarboxylic acids and their blends with citric acid. RSC Adv. 2016, 6, 88647–88655. [Google Scholar] [CrossRef]
- Son, D.H.; Bae, H.E.; Bae, M.J.; Lee, S.-H.; Cheong, I.W.; Park, Y.I.; Jeong, J.-E.; Kim, J.C. Fast, Localized, and Low-Energy Consumption Self-Healing of Automotive Clearcoats Using a Photothermal Effect Triggered by NIR Radiation. ACS Appl. Polym. Mater. 2022, 4, 3802–3810. [Google Scholar] [CrossRef]
- Cong, C.; Wang, J.; Wang, Z.; Xing, G.; Wang, Z. Photothermal healing performance of oxidized carbon black/epoxy vitrimer composite coating for metal protection. Prog. Org. Coat. 2023, 179, 107484. [Google Scholar] [CrossRef]
- Van Lijsebetten, F.; Engelen, S.; Bauters, E.; Van Vooren, W.; Smulders, M.M.J.; Du Prez, F.E. Recyclable vitrimer epoxy coatings for durable protection. Eur. Polym. J. 2022, 176, 111426. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L.; Feng, S.; Li, L. Bio-Based Vitrimeric Silicone Materials with High-Strength, Reprocessable, Healing, and Transparent Properties. Macromol. Rapid Commun. 2023, 44, 2300445. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Jin, C.; Chen, Z.; An, L.; Wang, T. On the Welding of Vitrimers: Chemistry, Mechanics and Applications. Adv. Funct. Mater. 2023, 33, 2300288. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, Z.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. Carbon nanotube–vitrimer composite for facile and efficient photo-welding of epoxy. Chem. Sci. 2014, 5, 3486–3492. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, J.; Zhao, H.; He, W.; Zhang, L.; Cheng, Z. Second-Generation Soft Actuators Driven by NIR Light Based on Croconaine Dye-Doped Vitrimers. ACS Appl. Mater. Interfaces 2023, 15, 41916–41926. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; Wei, Y.; Zhu, C. Recyclable and reformable epoxy resins based on dynamic covalent bonds—Present, past, and future. Polym. Test. 2022, 105, 107420. [Google Scholar] [CrossRef]
- Ma, J.; Porath, L.E.; Haque, M.F.; Sett, S.; Rabbi, K.F.; Nam, S.; Miljkovic, N.; Evans, C.M. Ultra-thin self-healing vitrimer coatings for durable hydrophobicity. Nat. Commun. 2021, 12, 5210. [Google Scholar] [CrossRef] [PubMed]
- Altuna, F.I.; Antonacci, J.; Arenas, G.F.; Pettarin, V.; Hoppe, C.E.; Williams, R.J.J. Photothermal triggering of self-healing processes applied to the reparation of bio-based polymer networks. Mater. Res. Express 2016, 3, 045003. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]
- Puig, J.; Hoppe, C.E.; Fasce, L.A.; Pérez, C.J.; Piñeiro-Redondo, Y.; Bañobre-López, M.; López-Quintela, M.A.; Rivas, J.; Williams, R.J.J. Superparamagnetic Nanocomposites Based on the Dispersion of Oleic Acid-Stabilized Magnetite Nanoparticles in a Diglycidylether of Bisphenol A-Based Epoxy Matrix: Magnetic Hyperthermia and Shape Memory. J. Phys. Chem. C 2012, 116, 13421–13428. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Eisa, M.H.; Maaza, M. Graphene Nanocomposites in Space Sector—Fundamentals and Advancements. C 2023, 9, 29. [Google Scholar] [CrossRef]
- Shepelev, O.; Kenig, S.; Dodiuk, H. Nanotechnology-based thermosets. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 833–890. ISBN 978-0-12-821632-3. [Google Scholar]
- Karak, N. Epoxy Nanocomposites with Carbon Nanotubes. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1385, pp. 169–200. [Google Scholar]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, Y.; Ji, Y. Carbon material/vitrimer composites: Towards sustainable, functional, and high-performance crosslinked polymeric materials. Giant 2023, 13, 100136. [Google Scholar] [CrossRef]
- Hubbard, A.M.; Ren, Y.; Papaioannou, P.; Sarvestani, A.; Picu, C.R.; Konkolewicz, D.; Roy, A.K.; Varshney, V.; Nepal, D. Vitrimer Composites: Understanding the Role of Filler in Vitrimer Applicability. ACS Appl. Polym. Mater. 2022, 4, 6374–6385. [Google Scholar] [CrossRef]
- Qiu, M.; Wu, S.; Tang, Z.; Guo, B. Exchangeable interfacial crosslinks towards mechanically robust elastomer/carbon nanotubes vitrimers. Compos. Sci. Technol. 2018, 165, 24–30. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X. Improving the transesterification and electrical conductivity of vitrimers by doping with conductive polymer wrapped carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2017, 99, 15–22. [Google Scholar] [CrossRef]
- Feng, Y.; Nie, Z.; Deng, P.; Luo, L.; Hu, X.; Su, J.; Li, H.; Fan, X.; Qi, S. An Effective Approach to Improve the Thermal Conductivity, Strength, and Stress Relaxation of Carbon Nanotubes/Epoxy Composites Based on Vitrimer Chemistry. Int. J. Mol. Sci. 2022, 23, 8833. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, A.G.; Dai, H.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene Pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- dell’Erba, I.E.; Hoppe, C.E.; Williams, R.J.J. Synthesis of Silver Nanoparticles Coated with OH-Functionalized Organic Groups: Dispersion and Covalent Bonding in Epoxy Networks. Langmuir 2010, 26, 2042–2049. [Google Scholar] [CrossRef]
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. (Eds.) Thermosetting Polymers; M. Dekker: New York, NY, USA, 2002. [Google Scholar]
- Altuna, F.; Hoppe, C.; Williams, R. Epoxy Vitrimers: The Effect of Transesterification Reactions on the Network Structure. Polymers 2018, 10, 43. [Google Scholar] [CrossRef]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Chappuis, S.; Edera, P.; Cloitre, M.; Tournilhac, F. Enriching an Exchangeable Network with One of Its Components: The Key to High-T g Epoxy Vitrimers with Accelerated Relaxation. Macromolecules 2022, 55, 6982–6991. [Google Scholar] [CrossRef]
- Poutrel, Q.-A.; Blaker, J.J.; Soutis, C.; Tournilhac, F.; Gresil, M. Dicarboxylic acid-epoxy vitrimers: Influence of the off-stoichiometric acid content on cure reactions and thermo-mechanical properties. Polym. Chem. 2020, 11, 5327–5338. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers with a covalently bonded tertiary amine as catalyst of the transesterification reaction. Eur. Polym. J. 2019, 113, 297–304. [Google Scholar] [CrossRef]
- Abdalla, M.; Dean, D.; Adibempe, D.; Nyairo, E.; Robinson, P.; Thompson, G. The effect of interfacial chemistry on molecular mobility and morphology of multiwalled carbon nanotubes epoxy nanocomposite. Polymer 2007, 48, 5662–5670. [Google Scholar] [CrossRef]
- Lin, Y.; Meziani, M.J.; Sun, Y.-P. Functionalized carbon nanotubes for polymeric nanocomposites. J. Mater. Chem. 2007, 17, 1143. [Google Scholar] [CrossRef]
- Chen, W.; Auad, M.L.; Williams, R.J.J.; Nutt, S.R. Improving the dispersion and flexural strength of multiwalled carbon nanotubes–stiff epoxy composites through β-hydroxyester surface functionalization coupled with the anionic homopolymerization of the epoxy matrix. Eur. Polym. J. 2006, 42, 2765–2772. [Google Scholar] [CrossRef]
- Auad, M.L.; Mosiewicki, M.A.; Uzunpinar, C.; Williams, R.J.J. Functionalization of carbon nanotubes and carbon nanofibers used in epoxy/amine matrices that avoid partitioning of the monomers at the fiber interface. Polym. Eng. Sci. 2010, 50, 183–190. [Google Scholar] [CrossRef]
- Chiu, W.-M.; Chang, Y.-A. Chemical modification of multiwalled carbon nanotube with the liquid phase method. J. Appl. Polym. Sci. 2008, 107, 1655–1660. [Google Scholar] [CrossRef]
- Pistone, A.; Ferlazzo, A.; Lanza, M.; Milone, C.; Iannazzo, D.; Piperno, A.; Piperopoulos, E.; Galvagno, S. Morphological Modification of MWCNT Functionalized with HNO3/H2SO4 Mixtures. J. Nanosci. Nanotechnol. 2012, 12, 5054–5060. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Avilés, F.; Ponce, A.; Cauich-Rodríguez, J.V.; Martínez, G.T. TEM Examination of MWCNTs Oxidized by Mild Experimental Conditions. Fuller. Nanotub. Carbon Nanostruct. 2012, 20, 49–55. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: Boston, MA, USA, 1991. [Google Scholar]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Teng, L.; Tang, T. IR study on surface chemical properties of catalytic grown carbon nanotubes and nanofibers. J. Zhejiang Univ. Sci. A 2008, 9, 720–726. [Google Scholar] [CrossRef]
- dell’Erba, I.E.; Martínez, F.D.; Hoppe, C.E.; Eliçabe, G.E.; Ceolín, M.; Zucchi, I.A.; Schroeder, W.F. Mechanism of Particle Formation in Silver/Epoxy Nanocomposites Obtained through a Visible-Light-Assisted in Situ Synthesis. Langmuir 2017, 33, 10248–10258. [Google Scholar] [CrossRef] [PubMed]
- Thi Mai Hoa, L. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4. Diam. Relat. Mater. 2018, 89, 43–51. [Google Scholar] [CrossRef]
- Leonardi, A.B.; Fasce, L.A.; Zucchi, I.A.; Hoppe, C.E.; Soulé, E.R.; Pérez, C.J.; Williams, R.J.J. Shape memory epoxies based on networks with chemical and physical crosslinks. Eur. Polym. J. 2011, 47, 362–369. [Google Scholar] [CrossRef]
- Yang, Y.; Terentjev, E.M.; Wei, Y.; Ji, Y. Solvent-assisted programming of flat polymer sheets into reconfigurable and self-healing 3D structures. Nat. Commun. 2018, 9, 1906. [Google Scholar] [CrossRef]




















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne Prudente, T.E.; Mauro, D.; Puig, J.; Altuna, F.I.; Da Ros, T.; Hoppe, C.E. Synthesis and Processing of Near Infrared—Activated Vitrimer Nanocomposite Films Modified with β-Hydroxyester-Functionalized Multi-Walled Carbon Nanotubes. C 2023, 9, 119. https://doi.org/10.3390/c9040119
Byrne Prudente TE, Mauro D, Puig J, Altuna FI, Da Ros T, Hoppe CE. Synthesis and Processing of Near Infrared—Activated Vitrimer Nanocomposite Films Modified with β-Hydroxyester-Functionalized Multi-Walled Carbon Nanotubes. C. 2023; 9(4):119. https://doi.org/10.3390/c9040119
Chicago/Turabian StyleByrne Prudente, Tomás E., Diandra Mauro, Julieta Puig, Facundo I. Altuna, Tatiana Da Ros, and Cristina E. Hoppe. 2023. "Synthesis and Processing of Near Infrared—Activated Vitrimer Nanocomposite Films Modified with β-Hydroxyester-Functionalized Multi-Walled Carbon Nanotubes" C 9, no. 4: 119. https://doi.org/10.3390/c9040119
APA StyleByrne Prudente, T. E., Mauro, D., Puig, J., Altuna, F. I., Da Ros, T., & Hoppe, C. E. (2023). Synthesis and Processing of Near Infrared—Activated Vitrimer Nanocomposite Films Modified with β-Hydroxyester-Functionalized Multi-Walled Carbon Nanotubes. C, 9(4), 119. https://doi.org/10.3390/c9040119