Investigating the Potential of Greener-Porous Graphene for the Treatment of Organic Pollutants in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Adsorbent Preparation
2.2. Analytical Methods
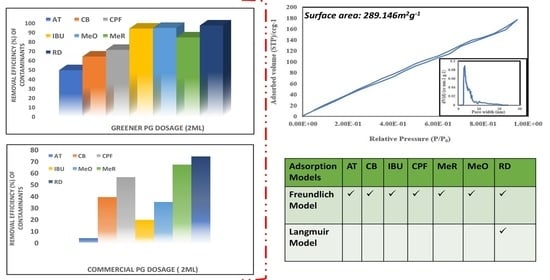
Microstructural Surface Area Characterization
2.3. Batch Tests and Analysis
2.3.1. Effect of the Contact Time and Study of Kinetic Models
2.3.2. Effect of the Adsorbent Dosage and Study of Adsorption Isotherms
3. Results and Discussion
3.1. Morphological Study of the Greener PG
3.2. Adsorption Performance
3.2.1. Effect of Contact Time on the Contaminants’ Removal
3.2.2. Effect of Various Adsorbent Dosages on Contaminant Removal
4. Conclusions and Outlook
- (i)
- Fast sorption kinetics are followed by a pseudo second-order model;
- (ii)
- With the increase in greener PG dosage, adsorption of the investigated contaminants increases;
- (iii)
- Langmuir model fitted best for rhodamine-b dye, confirming the monomolecular adsorption on the greener PG surface and no stacking of the adsorbed dye;
- (iv)
- The remaining six contaminants followed the Freundlich model, involving a possibility of physisorption;
- (v)
- Overall, removal efficiency of the greener PG showed better performance when compared with commercial rGO-obtained PG, for all the seven contaminants.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filho, W.L.; Ellams, D.; Han, S.; Tyler, D.; Boiten, V.J.; Paco, A.; Moora, H.; Balogun, A.L. A review of the socio-economic advantages of textile recycling. J. Clean. Prod. 2019, 218, 10–20. [Google Scholar] [CrossRef]
- Cooter, R.; Pickstone, J. (Eds.) Companion to Medicine in the Twentieth Century, 1st ed.; Routledge: Oxfordshire, UK, 2002. [Google Scholar] [CrossRef]
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 2018, 8, 1817. [Google Scholar] [CrossRef] [PubMed]
- UNICEF Division of Global Communication and Advocacy. Triple Threat How Disease, Climate Risks, and Unsafe Water, Sanitation and Hygiene Create a Deadly Combination for Children; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2023; Available online: www.unicef.org (accessed on 31 March 2023).
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Shetty, D.; Jahovic, I.; Raya, J.; Ravaux, F.; Jouiad, M.; Olsen, J.C.; Trabolsi, A. An ultra-absorbent alkyne-rich porous covalent polycalix[4]arene for water purification. J. Mater. Chem. A Mater. 2017, 5, 62–66. [Google Scholar] [CrossRef]
- Dutta, S.K.; Amin, M.K.; Ahmed, J.; Elias, M.; Mahiuddin, M. Removal of toxic methyl orange by a cost-free and eco-friendly adsorbent: Mechanism, phytotoxicity, thermodynamics, and kinetics. S. Afr. J. Chem. Eng. 2022, 40, 195–208. [Google Scholar] [CrossRef]
- Muthuraman, G.; Teng, T.T. Extraction of methyl red from industrial wastewater using xylene as an extractant. Prog. Nat. Sci. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of therapeutic drugs in the environment. Lancet 2000, 355, 1789–1790. [Google Scholar] [CrossRef]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef]
- Carpenter, C.M.G.; Helbling, D.E. Widespread Micropollutant Monitoring in the Hudson River Estuary Reveals Spatiotemporal Micropollutant Clusters and Their Sources. Environ. Sci. Technol. 2018, 52, 6187–6196. [Google Scholar] [CrossRef]
- Bottoni, P.; Caroli, S. Presence of residues and metabolites of pharmaceuticals in environmental compartments, food commodities and workplaces: A review spanning the three-year period 2014–2016. Microchem. J. 2018, 136, 2–24. [Google Scholar] [CrossRef]
- Schafhauser, B.H.; Kristofco, L.A.; de Oliveira, C.M.R.; Brooks, B.W. Global review and analysis of erythromycin in the environment: Occurrence, bioaccumulation and antibiotic resistance hazards. Environ. Pollut. 2018, 238, 440–451. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Bury, N.R.; Owen, S.F.; MacRae, J.I.; Barron, L.P. A review of the pharmaceutical exposome in aquatic fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C. Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal. Bioanal. Chem. 2007, 387, 1159–1162. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Daughton, C.G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rational for and avenues toward a green pharmacy. Environ. Health Perspect. 2003, 111, 757–774. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Salmon, D.; Zhang, S.; Butler, D. Nanostructured porous graphene for efficient removal of emerging contaminants (pharmaceuticals) from water. Chem. Eng. J. 2020, 398, 125440. [Google Scholar] [CrossRef]
- Del Rosario Brunetto, M.; Clavijo, S.; Delgado, Y.; Orozco, W.; Gallignani, M.; Ayala, C.; Cerdà, V. Development of a MSFIA sample treatment system as front end of GC-MS for atenolol and propranolol determination in human plasma. Talanta 2015, 132, 15–22. [Google Scholar] [CrossRef]
- Velasco, M.; Romero, B.; Betancourt, M.; Suarez, N.; Contreras, F. Uso de los Antagonistas Beta-Adrenérgicos en la Hipertensión Arterial. Arch. Venez. Farmacol. Ter. 2002, 21, 139–147. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798-02642002000200002&lng=es&nrm=iso&tlng=es (accessed on 9 August 2023).
- Meador, K.J.; Seliger, J.; Boyd, A.; Razavi, B.; Falco-Walter, J.; Le, S.; Loring, D.W. Comparative neuropsychological effects of carbamazepine and eslicarbazepine acetate. Epilepsy Behav. 2019, 94, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a complex mixture of therapeutic drugs at environmental levels on human embryonic cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Peart, T.E.; Svoboda, M.L. Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1139, 45–52. [Google Scholar] [CrossRef]
- Jiang, W.T.; Chang, P.H.; Wang, Y.S.; Tsai, Y.; Jean, J.S.; Li, Z.; Krukowski, K. Removal of ciprofloxacin from water by birnessite. J. Hazard. Mater. 2013, 250–251, 362–369. [Google Scholar] [CrossRef]
- Khan, S.J.; Wang, L.; Hashim, N.H.; Mcdonald, J.A. Distinct enantiomeric signals of ibuprofen and naproxen in treated wastewater and sewer overflow. Chirality 2014, 26, 739–746. [Google Scholar] [CrossRef]
- Lishman, L.; Smyth, S.A.; Sarafin, K.; Kleywegt, S.; Toito, J.; Peart, T.; Lee, B.; Servos, M.; Beland, M.; Seto, P. Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada. Sci. Total Environ. 2006, 367, 544–558. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Fenton, B.; Salmon, D.; Zhang, S.; Butler, D. Performance evaluation of porous graphene as filter media for the removal of pharmaceutical/emerging contaminants from water and wastewater. Nanomaterials 2021, 11, 79. [Google Scholar] [CrossRef]
- Han, L.; Khalil, A.M.E.; Wang, J.; Chen, Y.; Li, F.; Chang, H.; Zhang, H.; Liu, X.; Li, G.; Jia, Q.; et al. Graphene-boron nitride composite aerogel: A high efficiency adsorbent for ciprofloxacin removal from water. Sep. Purif. Technol. 2022, 278, 119605. [Google Scholar] [CrossRef]
- Ismail, Z. Green reduction of graphene oxide by plant extracts: A short review. Ceram. Int. 2019, 45, 23857–23868. [Google Scholar] [CrossRef]
- Fonseca, A.M.; Monte, F.J.Q.; de Oliveira, M.d.C.F.; de Mattos, M.C.; Cordell, G.A.; Braz-Filho, R.; Lemos, T.L.G. Coconut water (Cocos nucifera L.)—A new biocatalyst system for organic synthesis. J. Mol. Catal. B Enzym. 2009, 57, 78–82. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Qi, D.; Lin, J. Green Synthesis of Porous Spherical Reduced Graphene Oxide and Its Application in Immobilized Pectinase. ACS Omega 2020, 5, 32706–32714. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.Z.; Durmus, Z.; Sevgi, E. In situ reduction of graphene oxide by different plant extracts as a green catalyst for selective hydrogenation of nitroarenes. Int. J. Hydrogen Energy 2019, 44, 26322–26337. [Google Scholar] [CrossRef]
- Joshi, B.; Khalil, A.M.E.; Tabish, T.A.; Memon, F.A.; Chang, H.; Zhang, S. Near Green Synthesis of Porous Graphene from Graphite Using an Encapsulated Ferrate(VI) Oxidant. ACS Omega 2023, 8, 29674–29684. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, R.; Falyouna, O.; Bensaida, K.; Sugihara, Y.; Eljamal, O. Insights into kinetics, isotherms and thermodynamics of phosphorus sorption onto nanoscale zero-valent iron. J. Mol. Liq. 2021, 328, 115402. [Google Scholar] [CrossRef]
- Taguba, M.A.M.; Ong, D.C.; Ensano, B.M.B.; Kan, C.C.; Grisdanurak, N.; Yee, J.J.; de Luna, M.D.G. Nonlinear isotherm and kinetic modeling of cu(Ii) and pb(ii) uptake from water by MnFe2O4/chitosan nanoadsorbents. Water 2021, 13, 1662. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pu, C.; Wan, J.; Liu, E.; Yin, Y.; Li, J.; Ma, Y.; Fan, J.; Hu, X. Two-dimensional porous architecture of protonated GCN and reduced graphene oxide via electrostatic self-assembly strategy for high photocatalytic hydrogen evolution under visible light. Appl. Surf. Sci. 2017, 399, 139–150. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, L.; Zhu, J.; Chen, M.; Shi, W.; Xie, J. Novel p–n heterojunction photocatalyst constructed by porous graphite-like C3N4 and nanostructured BiOI: Facile synthesis and enhanced photocatalytic activity. J. Chem. Soc. Dalton Trans. 2013, 42, 15726–15734. [Google Scholar] [CrossRef]
- Zhong, Y.; Yuan, J.; Wen, J.; Li, X.; Xu, Y.; Liu, W.; Zhang, S.; Fang, Y. Earth-abundant NiS co-catalyst modified metal-free mpg-C3N4/CNT nanocomposites for highly efficient visible-light photocatalytic H2 evolution. Dalton Trans. 2015, 44, 18260–18269. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Creighton, M.; Chen, Y.; Hurt, R.; Külaots, I. Porous structures in stacked, crumpled and pillared graphene-based 3D materials. Carbon 2014, 66, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Larese-Casanova, P. Sorption of carbamazepine by commercial graphene oxides: A comparative study with granular activated carbon and multiwalled carbon nanotubes. J. Colloid Interface Sci. 2014, 426, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Putra, A.; Masriati, S.; Amran, A. The Association Structures and Sustainability of Methyl Red and Methylene Blue In Water Systems, A Nonionic Surfactants (Tween-40 And Tween-80) And Cyclohexane. Int. J. Progress. Sci. Technol. 2019, 17, 117–125. [Google Scholar]
- Mottram, L.F.; Forbes, S.; Ackley, B.D.; Peterson, B.R. Hydrophobic analogues of rhodamine B and rhodamine 101: Potent fluorescent probes of mitochondria in living C. elegans. Beilstein J. Org. Chem. 2012, 8, 2156–2165. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Gilmanova, L.H.; Makhmutova, A.R.; Rakhimbekova, A.; Stoikov, I.I. Highly selective binding of methyl orange dye by cationic water-soluble pillar[5]arenes. Org. Biomol. Chem. 2016, 14, 4233–4238. [Google Scholar] [CrossRef]
- Thomas, S.; Shanks, R.; Chandran, S. (Eds.) Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Perwitasari, D.S.; Ardian, Y.; Pracesa, Y.; Pangestu, M.A.; Sampe Tola, P. Langmuir and Freundlich Isotherm Approximation on Adsorption Mechanism of Chrome Waste by Using Tofu Dregs. Nusant. Sci. Technol. Proc. 2021, 106–112. [Google Scholar] [CrossRef]
- Dada, A.O., Olalekan. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, N.; Ullah, N.; Falath, W. Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: A review of recent advances. J. Environ. Manag. 2021, 287, 112360. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Bi, J.; Zheng, S.; Xu, Z.; Zhu, D.; Alvarez, P.J. Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry. Environ. Toxicol. Chem. 2010, 29, 2713–2719. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chen, J.; Lin, J.H. Preparation of pore-size tunable activated carbon derived from waste coffee grounds for high adsorption capacities of organic dyes. J. Environ. Chem. Eng. 2020, 8, 103929. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, S.; Turner, P.; Paton, K.R.; Reed, B.P.; Brennan, B.; Koziol, K.; Pollard, A.J. Gas physisorption measurements as a quality control tool for the properties of graphene/graphite powders. Carbon 2020, 167, 585–595. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Cai, N.; Larese-Casanova, P. Application of positively-charged ethylenediamine-functionalized graphene for the sorption of anionic organic contaminants from water. J. Environ. Chem. Eng. 2016, 4, 2941–2951. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem. Eng. J. 2016, 284, 687–697. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Kumara, A.V.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, G.; Qian, B.; Hu, C.; Wang, Y.; Qiu, J. Electrospun composites made of reduced graphene oxide and activated carbon nanofibers for capacitive deionization. Electrochim. Acta 2014, 137, 388–394. [Google Scholar] [CrossRef]
- Zaka, A.; Ibrahim, T.H.; Khamis, M.I.; Samara, F. Adsorption characteristics of diclofenac sodium onto graphene nanoplatelets. Desalination Water Treat. 2020, 206, 331–339. [Google Scholar] [CrossRef]
- Malakootian, M.; Faraji, M.; Malakootian, M.; Nozari, M. Ciprofloxacin removal from aqueous media by adsorption process: A systematic review and meta-analysis. Desalination Water Treat. 2021, 229, 252–282. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M. Removal of malachite green dye from aqueous solution using reduced graphene oxide as an adsorbent. Mater. Today Proc. 2021, 47, 1173–1182. [Google Scholar] [CrossRef]
- Floare-Avram, C.V.; Marincas, O.; Feher, I.; Covaciu, F.D.; Floare, C.G.; Lazar, M.D.; Magdas, D.A. Characterization of the Adsorption of Bisphenol A and Carbamazepine from Aqueous Solution on Graphene Oxide and Partially Reduced Graphene Oxide by High-Performance Liquid Chromatography (HPLC). Anal. Lett. 2023, 56, 272–285. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Rajendra Kumar, R.T. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and π–π interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Yao, Y.; Bing, H.; Feifei, X.; Xiaofeng, C. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound-Naproxen, carbamazepine and nonylphenol-on activated carbon. Water Res. 2008, 42, 2873–2882. [Google Scholar] [CrossRef]
- Pal, J.; Deb, M.K.; Deshmukh, D.K.; Verma, D. Removal of methyl orange by activated carbon modified by silver nanoparticles. Appl. Water Sci. 2013, 3, 367–374. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]











| Contaminant Adsorbed onto Greener PG | Pseudo First-Order | Pseudo Second-Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Slope | Intercept | Qe (mg/g) | K1 (1/mins) | R2 | Slope | Intercept | Qe (mg/g) | K2 (g/mg mins) | |
| AT | 0.54 | 0.028 | 1.89 | 6.621 | −0.0002 | 0.99 | 0.07 | 0.38 | 13.38 | 0.0020 |
| CB | 0.90 | −0.01 | 2.54 | 12.72 | −0.001 | 0.98 | 0.05 | 0.523 | 19.09 | 696.53 |
| CPF | 0.56 | −0.02 | 1.93 | 6.94 | −0.0002 | 0.99 | 0.04 | 0.11 | 21.38 | 3962.14 |
| IBU | 0.94 | −0.02 | 3.10 | 22.2 | −0.0001 | 0.98 | 0.03 | 0.73 | 27.11 | 1005.0 |
| MeO | 0.94 | −0.04 | 3.06 | 21.45 | −0.0003 | 0.99 | 0.03 | 0.21 | 32.70 | 0.0002 |
| MeR | 0.97 | −0.03 | 2.89 | 18.02 | −0.0003 | 0.99 | 0.03 | 0.20 | 30.59 | 4574.6 |
| RD | 0.82 | −0.03 | 3.89 | 49.27 | −0.0003 | 0.95 | 0.002 | 0.76 | 44.54 | 2582.82 |
| CONTAMINANT | Freundlich Isotherm Model | Langmuir Isotherm Model | Temkin Isotherm Model |
|---|---|---|---|
| ATENOLOL (AT) | Kf ({mg/g} {mg/L}1/m) = 1.82 m = 1/n = 1 R2 = 0.93 AIC = 40.89 | Qmax (mg/g) = 3.79 Ki (l/mg) = −0.24 RL = −0.697878596 R2 = 0.77 AIC = 26.90 | Kt (L/mg) = 0.90 Bt (J/mol) = −25.32 R2 = 0.92 AIC = 54.22 |
| CARBAMAZEPINE (CB) | Kf ({mg/g} {mg/L}1/m) = 2.31 m = 1/n = 1 R2 = 0.92 AIC = 44.94 | Qmax (mg/g) = 5.15 Ki (l/mg) = −9.77 RL = −0.010334966 R2 = 0.73 AIC = 42.8 | Kt (L/mg) = 0.083 Bt (J/mol) = −20.99 R2 = 0.98 AIC = 63.99 |
| IBUPROFEN (IBU) | Kf ({mg/g} {mg/L}1/m) = 2.764 m = 1/n = 1 R2 = 0.764 AIC = 48.51 | Qmax (mg/g) = 7.61 Ki (l/mg) = −0.0104 RL = 1.116444 R2 = 0.390 AIC = 49.39 | Kt (L/mg) = 0.04 Bt (J/mol) = −11.55 R2 = 0.89 AIC = 83.40 |
| CIPROFLOXIN (CPF) | Kf ({mg/g} {mg/L}1/m) = 6.79 m = 1/n = 1 R2 = 0.98 AIC = 35.91 | Qmax (mg/g) = 17.15 Ki (l/mg) = −0.003 RL = 1.037482 R2 = 0.95 AIC = 43.37 | Kt (L/mg) = 0.041 Bt (J/mol) = −13.26 R2 = 0.99 AIC = 77.76 |
| METHYL RED (MeR) | Kf ({mg/g} {mg/L}1/m) = 4227.29 m = 1/n = 1 R2 = 0.82 AIC = 121.44 | Qmax (mg/g) = 19.799 Ki (l/mg) = −0.0011 RL = 1.98879407 R2 = 0.46 AIC = 49.26 | Kt (L/mg) = 0.024 Bt (J/mol) = −9.911 R2 = 0.89 AIC = 87.26 |
| METHYL ORANGE (MeO) | Kf ({mg/g} {mg/L}1/m) = 17.44 m = 1/n = 1 R2 = 0.98 AIC = 45.73 | Qmax (mg/g) = 28.52 Ki (l/mg) = −0.00018 RL = 1.00178603 R2 = 0.92 AIC = 50.74 | Kt (L/mg) = 0.001 Bt (J/mol) = −5.31 R2 = 0.988 AIC = 104.06 |
| RHODAMINE-B (RD) | Kf ({mg/g} {mg/L}1/m) = 75.77 m = 1/n = 1 R2 = 0.98 AIC = 58.0 | Qmax (mg/g) = 155.54 Ki (l/mg) = 24197.99 RL = 1.03314 × 10−6 R2 = 0.94 AIC = 23.72 | Kt (L/mg) = 2.9 × e−10 Bt (J/mol) = −7.122 R2 = 0.98 AIC = 114.59 |
| ADSORBENT MATERIAL | BET SSA (m2/g) | Contaminants Tested for Adsorption/Absorbate | ADSORPTION CAPACITY (mg/g) | References | RAG of Synthesis Process R—Red A—Amber G—Green |
|---|---|---|---|---|---|
| Graphite | 4.5 | Carbamazepine—CB Methyl Orange—MeO | CB—3.65 ± 0.05 MeO—13.6 | [64,65,66] | G |
| Commercial Graphene | 15 | Carbamazepine—CB Methyl Orange—MeO Ciprofloxacin—CPF Atenolol—AT Ibuprofen—IBU | CPF—323 AT—<6 IBU—6.0 CB—22.8 ± 0.5 MeO—89.3 | [66,67,68,69] | A |
| Graphene Oxide (GO) | 38 | Carbamazepine—CB Methyl Orange—MeO Ciprofloxacin—CPF Atenolol—AT Ibuprofen—IBU Methyl Red—MeR Diclofenac—DCF Rhodamine b—RD Gemfibrozil—GEM | CPF—417.79 AT—7.598 GEM—2.981 IBU—10.01 DCF—3.65 CB—8.89 MeR—63.69 MeO—16.83 RD—0.54 | [31,67,70,71] | R |
| Chemically reduced Graphene Oxide (rGO) | 53 | Ciprofloxacin—CPF Carbamazepine—CB Methyl Orange—MeO Diclofenac sodium—DCFS Malachite Green—MG | CPF—18.2 DCFS—59.67 CBZ—55.13 MG—279.85 MeO—244 | [72,73,74,75,76,77] | R |
| Commercial Porous Graphene (PG) | 82.76 | Carbamazepine—CB Methyl Orange—MeO Methyl Red—MeR Ciprofloxacin—CPF Atenolol—AT Ibuprofen—IBU Rhodamine b—RD | CPF—11.34 CB—7.92 AT—0.84 IBU—3.976 MeO—7.06 MeR—13.48 RD—4.872 | This paper | A,R |
| Multi walled Carbon nanotube—MWCNT | 160 | Carbamazepine—CB Methyl Orange—MeO Ibuprofen—IBU Ciprofloxacin—CPF | CPF—1.745 CBU—108 MeO—27.6 IBU—186.5 | [68,73,78,79] | A,R |
| GREENER PG | 289.14 | Ciprofloxacin—CPF Methyl Orange—MeO Methyl Red—MeR Rhodamine b—RD Atenolol—AT Ibuprofen—IBU Carbamazepine—CB | CPF—28.596 CB—25.74 AT—19.29 IBU—37.65 MeO—37.16 MeR—84.75 RD—38.52 | This paper | G |
| Porous Graphene | 679 | Ciprofloxacin—CPF Rhodamine b—RD Atenolol—AT Ibuprofen—IBU Carbamazepine—CB Gemfibrozil—GEM Diclofenac—DCF | CPF—370.11 AT—2.738 GEM—4.604 IBU—47.85 DCF—41.23 CB—88.96 RD—16.6 × 104 | [31] | R |
| Activated Carbon | 1156 | Ciprofloxacin—CPF Diclofenac—DCF Carbamazepine—CB Ibuprofen—IBU Methyl Red—MeR | CPF—1.860 DCF—56.2 CB—3.2 MeR—10.0 IBU—12.6 | [68,80,81,82] | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, B.; Khalil, A.M.E.; Zhang, S.; Memon, F.A. Investigating the Potential of Greener-Porous Graphene for the Treatment of Organic Pollutants in Wastewater. C 2023, 9, 97. https://doi.org/10.3390/c9040097
Joshi B, Khalil AME, Zhang S, Memon FA. Investigating the Potential of Greener-Porous Graphene for the Treatment of Organic Pollutants in Wastewater. C. 2023; 9(4):97. https://doi.org/10.3390/c9040097
Chicago/Turabian StyleJoshi, Bhavya, Ahmed M. E. Khalil, Shaowei Zhang, and Fayyaz A. Memon. 2023. "Investigating the Potential of Greener-Porous Graphene for the Treatment of Organic Pollutants in Wastewater" C 9, no. 4: 97. https://doi.org/10.3390/c9040097
APA StyleJoshi, B., Khalil, A. M. E., Zhang, S., & Memon, F. A. (2023). Investigating the Potential of Greener-Porous Graphene for the Treatment of Organic Pollutants in Wastewater. C, 9(4), 97. https://doi.org/10.3390/c9040097