Application of Non-Saccharomyces Yeasts to Wine-Making Process
Abstract
:1. Introduction
2. Contribution of Non-Saccharomyces Yeast Reduction in the Ethanol Content of Wines
3. Contribution to Wine by Non-Saccharomyces Yeast
4. Non-Saccharomyces Strains as Glycosidase Producers for Vinification
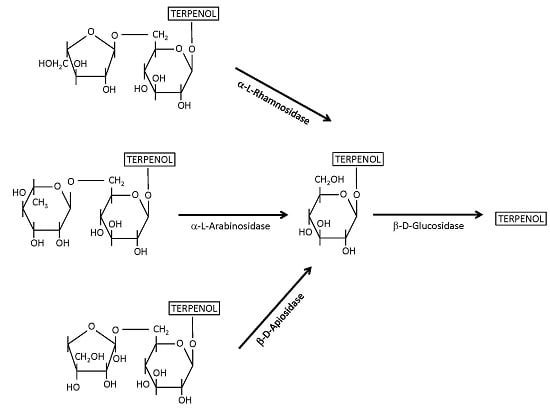
4.1. β-Glucosidases
4.2. Xylanases
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel Approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications, 1st ed.; Elsevier: San Diego, CA, USA, 1994. [Google Scholar]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of pure and mixed Cultures of the Main Wine yeast Species on Grape Must Fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Gil, J.V.; Mateo, J.J.; Jiménez, M.; Pastor, A.; Huerta, T. Aroma compounds in wine as influenced by apiculate yeasts. J. Food Sci. 1996, 61, 1247–1250. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Jiménez, M.; Huerta, T.; Pastor, A. Contribution of different yeasts isolated from musts of Monastrell grapes to the aroma of wine. Int. J. Food Microbiol. 1991, 14, 153–160. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.; Arroyo-López, F.N.; López-López, A.; Bautista-Gallego, J.; Garrido-Fernández, A. Lipolytic activity of the yeast species associated with the fermentation/storage phase of ripe olive processing. Food Microbiol. 2010, 27, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Heard, G. Novel yeasts in winemaking—Looking to the future. Food Aust. 1999, 51, 347–352. [Google Scholar]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Martini, A. Origin and domestication of the wine yeast Saccharomyces cerevisiae. J. Wine Res. 1993, 4, 165–176. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubordieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Johnson, E.A. Biotechnology of non-Saccharomyces yeasts—The Ascomycetes. Appl. Microbiol. Biotechnol. 2013, 97, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; Van Der Westhuizen, T.J.; Augustyn, O.P.H. Yeast biodiversity in vineyards and wineries and its importance to the South African wine industry—A review. S. Afr. J. Enol. Vitic. 1999, 20, 61–75. [Google Scholar]
- Kurtzman, C.P. The Yeasts: A Taxonomic Study, 5th ed.; Elsevier Science: Waltham, MA, USA, 2012. [Google Scholar]
- Davenport, R.R. Microecology of yeast and yeastlike organisms associated with and English vineyard. Vitis 1974, 13, 123–130. [Google Scholar]
- Jolly, N.P.; Vera, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Boynton, P.J.; Duncan, G. The ecology and evolution of non-domesticated Saccharomyces species. Yeast 2014, 12, 449–462. [Google Scholar]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of the wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.H.P.; Pretorius, I.S. The effect of non-Saccharomyces yeasts on fermentation and wine quality. S. Afr. J. Enol. Vitic. 2003, 24, 55–62. [Google Scholar]
- Romano, P.; Suzzi, G.; Domizio, P.; Fatichenti, F. Secondary products formation as a tool for discriminating non-Saccharomyces wine strains. Antonie Leeuwenhoek 1997, 71, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hebert, A.S.; Yan, X.; Zhao, Y.; Westphall, M.S.; Rush, M.J.P.; Zhu, G.; Champion, M.M.; Coon, J.J.; Dovichi, N.J. Over 10000 peptide identifications from the HeLa proteome by using single-shot capillary zone electrophoresis combined with tandem mass spectrometry. Angew. Chem. 2014, 126, 14151–14153. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Maturano, Y.P.; Rodríguez Assaf, L.A.; Toro, M.E.; Nally, M.C.; Vallejo, M.; Castellanos de Figueroa, L.I.; Combina, M.; Vazquez, F. Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int. J. Food Microbiol. 2012, 155, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kutyna, D.; Varela, C.; Henschke, P.; Chambers, P.; Stanley, G. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Technol. 2010, 21, 293–302. [Google Scholar] [CrossRef]
- Di Maio, S.; Polizzotto, G.; Di Gangi, E.; Foresta, G.; Genna, G.; Verzera, A. Biodiversity of indigenous Saccharomyces populations from old wineries of South-Eastern Sicily (Italy): Preservation and economic potential. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Gonzalez, R.; Quirós, M.; Morales, P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 2013, 29, 55–61. [Google Scholar] [CrossRef]
- Erten, H.; Tanguler, H. Influence of Williopsis saturnus yeasts in combination with Saccharomyces cerevisiae on wine fermentation. Lett. Appl. Microbiol. 2010, 50, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Tilloy, V.; Ortiz-Julien, A.; Dequin, S. Reduction of ethanol yield and improvement of glycerol formation by adaptive evolution of the wine yeast Saccharomyces cerevisiae under hyperosmotic conditions. Appl. Environ. Microbiol. 2014, 80, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Zironi, R.; Romano, P.; Suzzi, G.; Battistutta, F.; Comi, G. Volatile metabolites produced in wine by mixed and sequentialcultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol. Lett. 1993, 15, 235–238. [Google Scholar] [CrossRef]
- Arévalo-Villena, M.; Úbeda-Iranzo, J.F.; Gundllapalli, S.B.; Cordero-Otero, R.R.; Briones-Pérez, A.I. Characterization of an exocellular β-glucosidase from Debaryomyces pseudopolymorphus. Enzyme Microb. Technol. 2006, 39, 229–234. [Google Scholar] [CrossRef]
- Ocón, E.; Gutiérrez, A.R.; Garijo, P.; López, R.; Santamaría, P. Presence of non-Saccharomyces yeasts in cellar equipment and grape juice during harvest time. Food Microbiol. 2010, 27, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Jiménez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Arévalo-Villena, M.; Úbeda-Iranzo, J.F.; Briones-Pérez, A.I. β-Glucosidase activity in wine yeasts: Application in enology. Enzyme Microb. Technol. 2007, 40, 420–425. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles, Practice, Perception, 2nd ed.; Elsevier: San Diego, CA, USA, 2000. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma–A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Ubeda-Iranzo, J.F.; Briones-Perez, A.I.; Izquierdo-Cañas, P.M. Study of the oenological characteristics and enzymatic activities of wine yeasts. Food Microbiol. 1998, 15, 399–406. [Google Scholar] [CrossRef]
- Scanes, K.T.; Hohmann, S.; Prior, B.A. Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: A review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24. [Google Scholar]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Prior, B.A.; Toh, T.H.; Jolly, N.; Baccari, C.L.; Mortimer, R.K. Impact of yeast breeding for elevated glycerol production on fermentation activity and metabolite formation in Chardonnay. S. Afr. J. Enol. Vitic. 2000, 21, 92–99. [Google Scholar]
- Swiegers, J.H.; Kievit, R.L.; Siebert, T.; Lattey, K.A.; Bramley, B.R.; Francis, I.L. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 2009, 26, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Mateo, J.J.; Peris, L.; Ibañez, C.; Maicas, S. Characterization of glycolytic activities from non-Saccharomyces yeasts isolated from Bobal musts. J. Ind. Microbiol. Biotechnol. 2011, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Fleet, G.H.; Prakitchaiwattana, C.; Beh, A.L.; Heard, G. The yeast ecology of wine grapes. In Biodiversity and Biotechnology of Wine Yeasts; Ciani, M., Ed.; Research Signpost: Kerala, India, 2002; pp. 1–17. [Google Scholar]
- Strauss, M.L.; Jolly, N.P.; Lambrechts, M.G.; Rensburg, P.V. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Maicas, S.; Thieβen, C. Biotechnological characterisation of exocellular proteases produced by enological Hanseniaspora isolates. Int. J. Food Sci. Technol. 2015, 50, 218–225. [Google Scholar] [CrossRef]
- Pretorius, I.S. The genetic analysis and tailoring of wine yeasts. In Functional Genetics of Industrial Yeasts; De Winde, J.H., Ed.; Springer-Verlag: Berlin, Germany, 2003; Volume 2, pp. 99–142. [Google Scholar]
- Van Rensburg, P.; Stidwell, T.; Lambrechts, M.G.; Cordero-Otero, R.R.; Pretorius, I.S. Development and assessment of a recombinant Saccharomyces cerevisiae wine yeast producing two aroma-enhancing β-glucosidases encoded by the Saccharomycopsis fibuligera BGL1 and BGL2 genes. Anal. Microbiol. 2005, 55, 33–42. [Google Scholar]
- Yanai, T.; Sato, M. Isolation and properties of β-glucosidase produced by Debaryomyces hansenii and its application in winemakig. Am. J. Enol. Vitic. 1999, 50, 231–235. [Google Scholar]
- Sarry, J.E.; Gunata, Z. Plant and microbial glycoside hydrolases: Volatile release from glycosidic aroma precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Bayonove, C.; Gunata, Y.; Sapis, J.C.; Baumes, R.L.; Dugelay, I.; Grassin, C. L’aumento degli aromi nel vino mediante l’uso degli enzimi. Vignevini 1993, 9, 33–36. (In Italian) [Google Scholar]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Gunata, Z.; Bitteur, S.; Brillouet, J.M.; Bayonove, C.; Cordonnier, R. Sequential enzymatic hydrolysis of potentially aromatic glycosides form grapes. Carbohydr. Res. 1988, 184, 139–149. [Google Scholar] [CrossRef]
- Gunata, Z.; Bayonove, C.; Tapiero, C.; Cordonnier, R. Hydrolysis of grape monoterpenyl β-d-glucosides by various β-glucosidases. J. Agric. Food Chem. 1990, 38, 1232–1236. [Google Scholar] [CrossRef]
- Delcroix, A.; Gunata, Z.; Sapis, J.C.; Salmon, J.M.; Bayonove, C. Glycosidase activities of three enological yeast strains during wine making. Effect on the terpenol content of Muscat wine. Am. J. Enol. Vitic. 1994, 45, 291–296. [Google Scholar]
- Bisson, L.F.; Karpel, J.E. Genetics of yeast impacting wine quality. Ann. Rev. Food Sci. Technol. 2010, 1, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Gunata, Z.; Dugelay, I.; Sapis, J.C.; Baumes, R.; Bayonove, C. Action des glycosidases exogènes au cours de la vinification: Liberation de l’arôme à partir des précurseurs glycosidiques. J. Int. Sci. Vigne Vin 1990, 24, 133–144. (In French) [Google Scholar]
- Darriet, P.; Boidron, J.N.; Dubourdieu, D. L’hydrolyse des hétérosides terpéniques du Muscat a Petit Grains par les enzymes périplasmiques de Saccharomyces cerevisiae. Connaiss. Vigne Vin 1988, 22, 189–195. (In French) [Google Scholar]
- Mateo, J.J.; Di Stefano, R. Enological properties of β-glucosidase in wine yeasts. Food Microbiol. 1998, 14, 583–591. [Google Scholar] [CrossRef]
- Dupin, I.; Gunata, Z.; Sapis, J.C.; Bayonove, C.; M’Bairaroua, O.; Tapiero, C. Production of a β-apiosidase by Aspergillus niger. Partial purification, properties and effect on terpenyl apiosylglucosides from grape. J. Agric. Food Chem. 1992, 40, 1886–1891. [Google Scholar] [CrossRef]
- Spagna, G.; Barbagallo, R.N.; Palmeri, R.; Restuccia, C.; Giudici, P. Properties of endogenous β-glucosidase of a Saccharomyces cerevisiae strain isolated from Sicilian musts and wines. Enzyme Microb. Technol. 2002, 31, 1030–1035. [Google Scholar] [CrossRef]
- Cordero Otero, R.R.; Ubeda Iranzo, J.F.; Briones-Perez, A.I.; Potgieter, N.; Villena, M.A.; Pretorius, I.S.; van Rensburg, P. Characterization of the β-glucosidase activity produced by enological strains of non-Saccharomyces yeasts. J. Food Sci. 2003, 68, 2564–2569. [Google Scholar] [CrossRef]
- García, A.; Carcel, C.; Dulau, L.; Samson, A.; Aguera, E.; Agosin, E. Influence of a mixed culture with Debaryomyces vanriji and Saccharomyces cerevisiae on the volatiles of a Muscat wine. J. Food Sci. 2002, 67, 1138–1143. [Google Scholar] [CrossRef]
- Jutaporn, S.; Sukanda, V.; Christian, E.B.; Kanit, V. The characterisation of a novel Pichia anomala β-glucosidase with potentially aroma-enhancing capabilities in wine. Ann. Microbiol. 2009, 59, 335–343. [Google Scholar]
- Carrascosa, A.V.; Martinez-Rodriguez, A.; Cebollero, E.; Gonzalez, R. Molecular Wine Microbiology. In Saccharomyces Yeast II: Secondary Fermentation; Carrascosa, A.V., Muñoz, R., Gonzalez, R., Eds.; Elsevier: London, UK, 2011; pp. 33–49. [Google Scholar]
- Hernandez, L.F.; Espinosa, J.C.; Fernandez, G.M.; Briones, A. β-glucosidase activity in a Saccharomyces cerevisiae wine strain. Int. J. Food Microbiol. 2003, 80, 171–176. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, C.; Li, J.M.; Xu, Y. Different influences of β-glucosidases on volatile compounds and anthocyanins of Cabernet Gernischt and possible reason. Food Chem. 2013, 140, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Rapp, A.; Rieth, W. Enzymatische Freisetzung gebundener Aromastoffe in Wein. Dtsch. Lebensm. Rundsch. 1987, 83, 7–12. (In German) [Google Scholar]
- Gunata, Z.; Brillouet, J.M.; Voirin, S.; Baumes, B.; Cordonnier, R. Purification and some properties of an α-l-arabinofuranosidase from Aspergillus niger. Action on grape monoterpenyl arabinofuranosyl glucosidases. J. Agric. Food Chem. 1990, 38, 772–776. [Google Scholar] [CrossRef]
- Gueguen, Y.; Chemardin, P.; Pien, S.; Arnaud, A.; Galzy, P. Enhancement of aromatic quality of Muscat wine by the use of immobilized β-glucosidase. J. Biotechnol. 1997, 55, 151–156. [Google Scholar] [CrossRef]
- Brimer, L.; Nout, M.J.R.; Tuncel, G. Glycosidase (amygdalase and linamarase) from Endomyces fibuliger (LU677): Formation and crude enzyme properties. Appl. Microbiol. Biotechnol. 1998, 49, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of β-glucosidase activity in yeasts of oenological origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R.; Vinella, M.; Domizio, P.; Zito, F.; Sanseverino, F.; D’Elia, A.; Rosi, I. Extraction and immobilization in one step of two β-glucosidases released from a yeast strain of Debaryomyces hansenii. Enzyme Microb. Technol. 1999, 24, 123–129. [Google Scholar] [CrossRef]
- Fernández-González, M.; Di Stefano, R.; Briones, A.I. Hydrolysis and transformation of terpene glycosides from Muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- Mendes, A.; Climaco, M.C.; Mendes, A. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components–A preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef]
- Madrigal, T.; Maicas, S.; Mateo, J.J. Glucose and ethanol tolerant enzymes produced by Pichia (Wickerhamomyces) isolates from enological ecosystems. Am. J. Enol. Vitic. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Lopez, S.; Mateo, J.J.; Maicas, S. Characterization of Hanseniaspora isolates with potential aroma enhancing properties in Muscat wines. S. Afr. J. Enol. Vitic. 2014, 35, 292–303. [Google Scholar]
- Wang, Y.; Xu, Y.; Li, J. A Novel extracellular β-glucosidase from Trichosporon asahii: Yield prediction, evaluation and application for aroma enhancement of cabernet sauvignon. J. Food Sci. 2012, 77, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qin, Y.; Tao, Y.S.; Zhu, X.L.; Peng, C.T.; Ullah, N. Potential of glycosidase from non-Saccharomyces isolates for enhancement of wine aroma. J. Food Sci. 2016, 81, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.K.; Hazlewood, G.P. Enzymology and other characteristics of cellulases and xylanases. In Enzymes in Farm Animal Nutrition; Bedford, M., Partridge, G., Eds.; CABI Publishing: Oxon, UK, 2001. [Google Scholar]
- Polizeli, M.L.; Rizzati, A.C.; Monti, R.H.F.; Terenzi, C.G.; Jorge, J.A.; Amorin, D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Linden, T.; Hahn-Hagerdal, B. Fermentation of lignocellulose hydrolysates with yeasts and xylose isomerase. Enzyme Microb. Technol. 1989, 11, 583589. [Google Scholar] [CrossRef]
- López, M.C.; Mateo, J.J.; Maicas, S. Screening of β-glucosidase and β-xylosidase activities in four non-Saccharomyces yeast isolates. J. Food Sci. 2015, 80, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Mateo, J.J.; Maicas, S. Characterization of an ethanol-tolerant 1,4-β-xylosidase produced by Pichia membranifaciens. Lett. Appl. Microbiol. 2012, 55, 354–361. [Google Scholar] [CrossRef] [PubMed]

| Aureobasidium pullulans | Hansenula sp |
| Brettanomyces sp | Issatchenkia terricola |
| B. anomalus | Kluyveromyces thermotolerans |
| Candida guilliermondii | Lachancea thermotolerans |
| C. molischiana | Metschnikowia pulcherrima/C. pulcherrima |
| C. stellata | Pichia angusta |
| C. utilis | P. anomala |
| C. zemplinina | P. capsulata |
| Debaryomyces castellii | P. guilliermondii |
| D.hansenii | P. kluyvery |
| D.polymorphus | P. membranifaciens |
| D.pseudopolymorphus | Saccharomycodes ludwigii |
| D. vanriji | Schizosaccharomyces pombe |
| Hanseniaspora sp. (Kloeckera) | Sporidiobolus pararoseus |
| H. guilliermondii | Torulaspora delbrueckii |
| H. osmophila | Trichosporon asahii |
| H. vineae | Wickerhamomyces anomalus |
| H. uvarum | Zygosaccharomyces bailii |
| Compound | Control b | Hanseniaspora Inoculated | ||
|---|---|---|---|---|
| H. uvarum H107 | H. vineae G26 | H. vineae P38 | ||
| Oxide A c | 29.7 (1.2) | 30.4 (2.1) | 33.7 (3.2) | 26.9 (3.4) |
| Oxide B d | nd | nd | nd | nd |
| Linalool | 20.0 (0.9) | 40.4 * (3.9) | 47.4 * (3.4) | 38.2 * (5.3) |
| Ho-trienol | 24.0 (3.2) | 51.3 *(5.3) | 35.1 * (4.2) | 24.9 * (0.6) |
| 2-Phenylethanol | 1890.2 (43.4) | 3057.5 * (39.8) | 2747.8 * (26.8) | 2568.5 * (45.6) |
| Oxide C e | nd | nd | nd | nd |
| Oxide D f | nd | nd | nd | nd |
| Terpineol | 53.3 (3.4) | 67.2 * (4.7) | 65.1 *(1.2) | 54.5 (3.9) |
| Nerol | 24.6 (2.8) | 25.8 (1.1) | 23.4 (3.1) | 26.3 (1.2) |
| Geraniol | 59.8 (5.0) | 61.3 (3.7) | 56.9 (1.7) | 62.8 (1.7) |
| Diol 1 g | 43.2 (4.7) | 87.9 * (2.1) | 80.2 * (2.1) | 81.2 * (3.2) |
| 4-Vinylphenol | 63.2 (1.2) | 89.7 * (2.4) | 75.7 * (5.8) | 62.1 (0.9) |
| Endiol h | nd | 58.8 * (2.1) | 52.0 * (3.4) | 34.1 * (4.2) |
| Diol 2 i | 12.0 (0.6) | 13.4 (0.9) | 7.8 (2.6) | 10.1 (0.9) |
| 2-Phenylethyl acetate | 28.0 (4.1) | 56.2 * (7.2) | 23.3 (1.2) | 25.8 (4.7) |
| 2-Methoxy-4-vinylphenol | 89.0 (6.1) | 103.0 * (5.3) | 105.4 * (6.5) | 94.1 (2.9) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo, J.J.; Maicas, S. Application of Non-Saccharomyces Yeasts to Wine-Making Process. Fermentation 2016, 2, 14. https://doi.org/10.3390/fermentation2030014
Mateo JJ, Maicas S. Application of Non-Saccharomyces Yeasts to Wine-Making Process. Fermentation. 2016; 2(3):14. https://doi.org/10.3390/fermentation2030014
Chicago/Turabian StyleMateo, José Juan, and Sergi Maicas. 2016. "Application of Non-Saccharomyces Yeasts to Wine-Making Process" Fermentation 2, no. 3: 14. https://doi.org/10.3390/fermentation2030014
APA StyleMateo, J. J., & Maicas, S. (2016). Application of Non-Saccharomyces Yeasts to Wine-Making Process. Fermentation, 2(3), 14. https://doi.org/10.3390/fermentation2030014