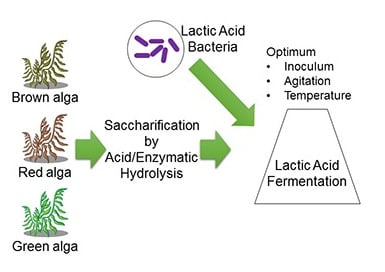
Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Compositions of Dried Seaweeds Gracilaria sp., S. siliquosum, and U. lactuca
2.2. Effect of Acid Concentration and Hydrolysis Time on the Dilute Acid Pretreatment of Gracilaria sp., S. siliquosum, and U. lactuca
2.3. The Effect of Cellulase Hydrolysis on Marine Algae Saccharification
2.4. The Effects of Inoculum, Agitation, and Temperature on Lactic Acid Fermentation
2.5. Optimum Conditions for Lactic Acid Fermentation
3. Materials and Methods
3.1. Source of Marine Algae and Lactic Acid Bacteria
3.2. Preparation of the Marine Algae Hydrolysate and Lactic Acid Fermentation
3.3. Reducing Sugars Quantification Using DNS (3,5-Dinitrosalicylic Acid) Assay
3.4. Analysis of Lactic Acid Using HPLC
3.5. Effect of Inoculum, Agitation, and Temperature on Lactic Acid Fermentation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-product lactic acid bacteria fermentations: A review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Liaud, N.; Rosso, M.N.; Fabre, N.; Crapart, S.; Herpoel-Gimbert, I.; Sigoillot, J.C.; Raouche, S.; Levasseur, A. L-lactic acid production by Aspergillus brasiliensis overexpressing the heterologous ldha gene from Rhizopus oryzae. Microb. Cell. Fact. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Tsai, S.P.; Bonsignore, P.; Moon, S.H.; Frank, J.R. Technological and economic-potential of poly (lactic acid) and lactic-acid derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Maas, R.H.; Bakker, R.R.; Eggink, G.; Weusthuis, R.A. Lactic acid production from xylose by the fungus Rhizopus oryzae. Appl. Microbiol. Biotechnol. 2006, 72, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.H.; Springer, J.; Eggink, G.; Weusthuis, R.A. Xylose metabolism in the fungus Rhizopus oryzae: Effect of growth and respiration on L(+)-lactic acid production. J. Ind. Microbiol. Biotechnol. 2008, 35, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T.; Tanaka, R. Mass production of spores of lactic acid-producing Rhizopus oryzae NBRC 5384 on agar plate. Biotechnol. Prog. 2013, 29, 876–881. [Google Scholar] [CrossRef]
- Ilmen, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttila, M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Liu, J.; Xu, H.; Li, W.; Zhang, J. L-lactic acid fermentation by Enterococcus faecium: A new isolate from bovine rumen. Biotechnol. Lett. 2015, 37, 1379–1383. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- de Lima, C.J.B.; Coelho, L.F.; Contiero, J. The use of response surface methodology in optimization of lactic acid production: Focus on medium supplementation, temperature and pH control. Food Technol. Biotechnol. 2010, 48, 175–181. [Google Scholar]
- Nuzzo, G.; Landi, S.; Esercizio, N.; Manzo, E.; Fontana, A.; d’Ippolito, G. Capnophilic lactic fermentation from Thermotoga neapolitana: A resourceful pathway to obtain almost enantiopure l-lactic acid. Fermentation 2019, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Ishida, N.; Saitoh, S.; Tokuhiro, K.; Nagamori, E.; Matsuyama, T.; Kitamoto, K.; Takahashi, H. Efficient production of L-lactic acid by metabolically engineered Saccharomyces cerevisiae with a genome-integrated L-lactate dehydrogenase gene. Appl. Environ. Microbiol. 2005, 71, 1964–1970. [Google Scholar] [CrossRef] [Green Version]
- Wakai, S.; Yoshie, T.; Asai-Nakashima, N.; Yamada, R.; Ogino, C.; Tsutsumi, H.; Hata, Y.; Kondo, A. L-lactic acid production from starch by simultaneous saccharification and fermentation in a genetically engineered Aspergillus oryzae pure culture. Bioresour. Technol. 2014, 173, 376–383. [Google Scholar] [CrossRef]
- Koivuranta, K.T.; Ilmen, M.; Wiebe, M.G.; Ruohonen, L.; Suominen, P.; Penttila, M. L-lactic acid production from D-xylose with Candida sonorensis expressing a heterologous lactate dehydrogenase encoding gene. Microb. Cell. Fact. 2014, 13, 107. [Google Scholar] [CrossRef] [Green Version]
- Adsul, M.G.; Varma, A.J.; Gokhale, D.V. Lactic acid production from waste sugarcane bagasse derived cellulose. Green Chem. 2007, 9, 58–62. [Google Scholar] [CrossRef]
- Okano, K.; Zhang, Q.; Shinkawa, S.; Yoshida, S.; Tanaka, T.; Fukuda, H.; Kondo, A. Efficient production of optically pure D-lactic acid from raw corn starch by using a genetically modified L-lactate dehydrogenase gene-deficient and alpha-amylase-secreting Lactobacillus plantarum strain. Appl. Environ. Microbiol. 2009, 75, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Mueller, R.E.; Jaeger, S.; Bajpai, R.; Lannotti, E.L. Lactic acid production from enzyme-thinned corn starch using Lactobacillus amylovorus. J. Ind. Microbiol. 1991, 7, 27–34. [Google Scholar] [CrossRef]
- Yin, P.M.; Nishina, N.; Kosakai, Y.; Yahiro, K.; Park, Y.; Okabe, M. Enhanced production of L(+)-lactic acid from corn starch in a culture of Rhizopus oryzae using an air-lift bioreactor. J. Ferment. Bioeng. 1997, 84, 249–253. [Google Scholar] [CrossRef]
- Fukushima, K.; Sogo, K.; Miura, S.; Kimura, Y. Production of D-lactic acid by bacterial fermentation of rice starch. Macromol. Biosci. 2004, 4, 1021–1027. [Google Scholar] [CrossRef]
- Huang, L.P.; Jin, B.; Lant, P.; Zhou, J.T. Simultaneous saccharification and fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Biochem. Eng. J. 2005, 23, 265–276. [Google Scholar] [CrossRef]
- Anuradha, R.; Suresh, A.K.; Venkatesh, K.V. Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochem. 1999, 35, 367–375. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Mandl, M.; Venus, J. Production and purification of l-lactic acid in lab and pilot scales using sweet sorghum juice. Fermentation 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Qiao, Q.G.; Chu, D.Q.; Gu, H.Q.; Dao, T.H.; Zhang, J.; Bao, J. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol. 2013, 135, 481–489. [Google Scholar] [CrossRef]
- Gullon, B.; Yanez, R.; Alonso, J.L.; Parajo, J.C. L-lactic acid. production from apple pomace by sequential hydrolysis and fermentation. Bioresour. Technol. 2008, 99, 308–319. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, X.R.; Qi, B.K.; Luo, J.Q.; Shen, F.; Su, Y.; Khan, R.; Wan, Y.H. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour. Technol. 2014, 163, 160–166. [Google Scholar] [CrossRef]
- Maas, R.H.W.; Bakker, R.R.; Jansen, M.L.A.; Visser, D.; De Jong, E.; Eggink, G.; Weusthuis, R.A. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: Neutralization of acid by fed-batch addition of alkaline substrate. Appl. Microbiol. Biotechnol. 2008, 78, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Wee, Y.J.; Yun, J.S.; Park, D.H.; Ryu, H.W. Biotechnological production of L(+)-lactic acid from wood hydrolyzate by batch fermentation of Enterococcus faecalis. Biotechnol. Lett. 2004, 26, 71–74. [Google Scholar] [CrossRef]
- Dominguez-Faus, R.; Powers, S.E.; Burken, J.G.; Alvarez, P.J. The water footprint of biofuels: A drink or drive issue? Environ. Sci. Technol. 2009, 43, 3005–3010. [Google Scholar] [CrossRef] [Green Version]
- Sebayang, A.H.; Masjuki, H.H.; Ong, H.C.; Dharma, S.; Silitonga, A.S.; Mahlia, T.M.I.; Aditiya, H.B. A perspective on bioethanol production from biomass as alternative fuel for spark ignition engine. RSC Adv. 2016, 6, 14964–14992. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- Enriquez, S.; Duarte, C.M.; SandJensen, K.; Nielsen, S.L. Broad-scale comparison of photosynthetic rates across phototrophic organisms. Oecologia 1996, 108, 197–206. [Google Scholar] [CrossRef]
- Sharif Hossain, A.B.M.; Salleh, A. Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar]
- Kim, S.K.; Chojnacka, K. (Eds.) Marine Algae Extracts: Processes, Products, and Applications; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Uchida, M.; Miyoshi, T. Algal fermentation-the seed for a new fermentation industry of foods and related products. JARQ Jpn. Agric. Res. Q. 2013, 47, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.J.; Lee, S.Y.; Kim, S.M.; Lee, S.B. Fermentation of seaweed sugars by Lactobacillus species and the potential of seaweed as a biomass feedstock. Biotechnol. Bioprocess Eng. 2011, 16, 1231–1239. [Google Scholar] [CrossRef]
- Wu, C.H.; Chien, W.C.; Chou, H.K.; Yang, J.; Lin, H.T.V. Sulfuric acid hydrolysis and detoxification of red alga Pterocladiella capillacea for bioethanol fermentation with thermotolerant yeast Kluyveromyces marxianus. J. Microbiol. Biotechnol. 2014, 24, 1245–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Hong, J.Y.; Jang, H.C.; Oh, S.G.; Kim, S.H.; Yoon, J.J.; Kim, Y.J. Use of Gelidium amansii as a promising resource for bioethanol: A practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour. Technol. 2012, 108, 83–88. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernandez, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Jang, J.S.; Cho, Y.; Jeong, G.T.; Kim, S.K. Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica. Bioprocess Biosyst. Eng. 2012, 35, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.H.; Xu, Z.; Zhang, W.Y.; Xiang, J. Effect of fermentation conditions on l-lactic acid production from soybean straw hydrolysate. J. Microbiol. Biotechnol. 2015, 25, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.K.; Jeong, G.T. Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol. Prog. 2005, 21, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Saha, B.C.; Hector, R.E.; Cotta, M.A. Removal of fermentation inhibitors from alkaline peroxide pretreated and enzymatically hydrolyzed wheat straw: Production of butanol from hydrolysate using Clostridium beijerinckii in batch reactors. Biomass Bioenerg. 2008, 32, 1353–1358. [Google Scholar] [CrossRef]
- Nancib, A.; Diboune, N.; Nancib, N.; Boudrant, J. Statistical optimization of dilute acid hydrolysis of wood sawdust for lactic acid production. J. Appl. Biotech. Bioeng. 2017, 4, 502–509. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Wood, B.J.B. Lactic Acid Bacteria: Biodiversity and Taxonomy; Wiley Blackwell: Chichester, West Sussex, UK, 2014. [Google Scholar] [CrossRef]
- Djukic-Vukovic, A.P.; Mojovic, L.V.; Vukasinovic-Sekulic, M.S.; Rakin, M.B.; Nikolic, S.B.; Pejin, J.D.; Bulatovic, M.L. Effect of different fermentation parameters on L-lactic acid production from liquid distillery stillage. Food Chem. 2012, 134, 1038–1043. [Google Scholar] [CrossRef]
- Salminen, S. Lactic Acid Bacteria: Microbiological and Functional Aspects, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2012; p. 777. [Google Scholar]
- Wardani, S.K.; Cahyanto, M.N.; Rahayu, E.S.; Utami, T. The effect of inoculum size and incubation temperature on cell growth, acidproduction and curd formation during milk fermentation by Lactobacillus plantarum Dad 13. Int. Food Res. J. 2017, 24, 921–926. [Google Scholar]
- Quemener, B.; Lahaye, M. Comparative analysis of sulfated galactans from red algae by reductive hydrolysis and mild methanolysis coupled to two different HPLC techniques. J. Appl. Phycol. 1998, 10, 75–81. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.S.; Hwang, H.J.; Park, M.S.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Production of l-lactic acid from a green microalga, Hydrodictyon reticulum, by Lactobacillus paracasei LA104 isolated from the traditional Korean food, makgeolli. Bioresour. Technol. 2012, 110, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the AOAC, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998. [Google Scholar]
- Harrigan, W.F.; McCance, M.E. Laboratory Methods in Food and Dairy Microbiology, Rev. ed.; Academic Press: Cambridge, MA, USA, 1976; p. 452. [Google Scholar]
- Trontel, A.; Batusic, A.; Gusic, I.; Slavica, A.; Santek, B.; Novak, S. Production of D- and L-Lactic Acid by Mono- and mixed cultures of Lactobacillus sp. Food Technol. Biotechnol. 2011, 49, 75–82. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Jang, S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Production of L(+)-lactic acid from mixed acid and alkali hydrolysate of brown seaweed. Food Sci. Technol. Res. 2011, 17, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Gardner, N.J.; Savard, T.; Obermeier, P.; Caldwell, G.; Champagne, C.P. Selection and characterization of mixed starter cultures for lactic acid fermentation of carrot, cabbage, beet and onion vegetable mixtures. Int. J. Food Microbiol. 2001, 64, 261–275. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; Gonzalez, J.M.D.; Converti, A.; Oliveira, R.P.D. Lactic acid properties, applications and production: A review. Trends Food Sci. Tech. 2013, 30, 70–83. [Google Scholar] [CrossRef]

| Components | Gracilaria sp. (%) | S. siliquosum (%) | U. lactuca (%) |
|---|---|---|---|
| Moisture | 7.54 ± 0.42 b | 13.83 ± 0.61 | 11.29 ± 0.21 |
| Carbohydrate a | 64.69 ± 0.32 | 63.92 ± 0.33 | 43.91 ± 0.42 |
| Crude Protein | 11.86 ± 0.16 | 9.61 ± 0.35 | 21.54 ± 0.06 |
| Crude fat | 1.42 ± 0.05 | 0.56 ± 0.05 | 0.51 ± 0.02 |
| Ash | 14.49 ± 1.23 | 12.08 ± 0.85 | 22.75 ± 0.37 |
| Marine Algae | Heating Time (min) | Acid Conc. (N) | Reducing Sugar Content (g/L) | Reducing Sugar Yield (%) |
|---|---|---|---|---|
| Gracilaria | ||||
| 20 | 0.2 | 13.30 ± 0.86 d | 20.56 ± 1.33 d | |
| 0.4 | 21.30 ± 0.47 b | 32.93 ± 0.73 b | ||
| 30 | 0.2 | 14.60 ± 0.75 d | 22.57 ± 1.16 d | |
| 0.4 | 23.32 ± 0.26 a | 36.05 ± 0.40 a | ||
| 60 | 0.2 | 17.38 ± 0.55 c | 26.87 ± 0.85 c | |
| 0.4 | 24.78 ± 0.75 a | 38.31 ± 1.16 a | ||
| S. siliquosum | ||||
| 20 | 0.2 | 4.19 ± 0.09 f | 6.56 ± 0.13 f | |
| 0.4 | 10.65 ± 0.16 c | 16.66 ± 0.25 c | ||
| 30 | 0.2 | 5.48 ± 0.48 e | 8.57 ± 0.75 e | |
| 0.4 | 12.18 ± 0.17 b | 19.06 ± 0.27 b | ||
| 60 | 0.2 | 7.86 ± 0.18 d | 12.30 ± 0.28 d | |
| 0.4 | 13.15 ± 0.23 a | 20.57 ± 0.36 a | ||
| U. lactuca | ||||
| 20 | 0.2 | 8.83 ± 0.12 d | 20.11 ± 0.27 d | |
| 0.4 | 16.26 ± 0.28 b | 37.03 ± 0.64 b | ||
| 30 | 0.2 | 8.31 ± 0.56 d | 18.93 ± 1.28 d | |
| 0.4 | 18.89 ± 0.39 a | 40.06 ± 0.89 a | ||
| 60 | 0.2 | 12.38 ± 0.10 c | 28.19 ± 0.23 c | |
| 0.4 | 18.72 ± 0.18 a | 42.63 ± 0.41 a |
| Seaweeds | Hydrolysis Steps | Reducing Sugar Content (g/L) | Reducing Sugar Yield (%) |
|---|---|---|---|
| Gracilaria sp. | |||
| Acid extraction | 21.71 ± 0.84 | 33.56 ± 1.29 | |
| Cellulase hydrolysis | 31.13 ± 1.59 | 48.12 ± 2.45 | |
| S. siliquosum | |||
| Acid extraction | 12.75 ± 0.51 | 19.95 ± 0.76 | |
| Cellulase hydrolysis | 19.51 ± 1.68 | 30.53 ± 2.60 | |
| U. lactuca | |||
| Acid extraction | 12.89 ± 2.51 | 27.34 ± 5.75 | |
| Cellulase hydrolysis | 18.99 ± 1.59 | 40.27 ± 3.63 |
| Inoculum (%, v/v) | LAB Count (log CFU/mL) | Reducing Sugar Content (g/L) | pH Value | Lactic Acid Concentration (g/L) |
|---|---|---|---|---|
| 1 | 8.00 ± 0.05 | 10.50 ± 0.99 | 4.20 ± 0.15 | 14.57 ± 0.98 a |
| 3 | 8.25 ± 0.48 | 10.69 ± 1.74 | 4.14 ± 0.18 | 14.87 ± 0.72 a |
| 6 | 8.40 ± 0.34 | 10.54 ± 1.29 | 4.11 ± 0.22 | 15.02 ± 0.80 a |
| 9 | 8.49 ± 0.20 | 12.10 ± 1.61 | 4.20 ± 0.19 | 14.54 ± 0.43 a |
| 12 | 8.51 ± 0.19 | 13.80 ± 2.34 | 4.37 ± 0.41 | 13.95 ± 1.29 a |
| 15 | 8.59 ± 0.12 | 14.52 ± 2.50 | 4.56 ± 0.44 | 13.24 ± 1.13 a |
| 18 | 8.62 ± 0.16 | 13.31 ± 1.29 | 4.34 ± 0.26 | 13.71 ± 1.62 a |
| Shaking Incubation (rpm) | LAB Count (log CFU/mL) | Reducing Sugar Content (g/L) | pH Value | Lactic Acid Concentration (g/L) |
|---|---|---|---|---|
| 0 | 8.94 ± 0.11 | 12.75 ± 0.16 | 4.26 ± 0.02 | 15.08 ± 0.25 a |
| 50 | 8.88 ± 0.13 | 12.75 ± 0.25 | 4.35 ± 0.03 | 14.63 ± 0.10 b |
| 100 | 8.77 ± 0.10 | 15.01 ± 0.38 | 4.77 ± 0.05 | 12.48 ± 0.04 c |
| 150 | 8.79 ± 0.14 | 14.77 ± 0.30 | 4.96 ± 0.01 | 12.62 ± 0.19 c |
| Fermentation Temperature (°C) | LAB Count (log CFU/mL) | Reducing Sugar Content (g/L) | pH Value | Lactic Acid Concentration (g/L) |
|---|---|---|---|---|
| 26 | 8.42 ± 0.23 | 13.73 ± 0.12 | 4.99 ± 0.02 | 13.39 ± 0.19 b |
| 30 | 9.23 ± 0.11 | 12.55 ± 0.04 | 4.43 ± 0.03 | 14.21 ± 0.16 a |
| 37 | 9.03 ± 0.05 | 12.50 ± 0.37 | 4.41 ± 0.07 | 14.24 ± 0.14 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-T.V.; Huang, M.-Y.; Kao, T.-Y.; Lu, W.-J.; Lin, H.-J.; Pan, C.-L. Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation 2020, 6, 37. https://doi.org/10.3390/fermentation6010037
Lin H-TV, Huang M-Y, Kao T-Y, Lu W-J, Lin H-J, Pan C-L. Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation. 2020; 6(1):37. https://doi.org/10.3390/fermentation6010037
Chicago/Turabian StyleLin, Hong-Ting Victor, Mei-Ying Huang, Te-Yu Kao, Wen-Jung Lu, Hsuan-Ju Lin, and Chorng-Liang Pan. 2020. "Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation" Fermentation 6, no. 1: 37. https://doi.org/10.3390/fermentation6010037
APA StyleLin, H. -T. V., Huang, M. -Y., Kao, T. -Y., Lu, W. -J., Lin, H. -J., & Pan, C. -L. (2020). Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation, 6(1), 37. https://doi.org/10.3390/fermentation6010037