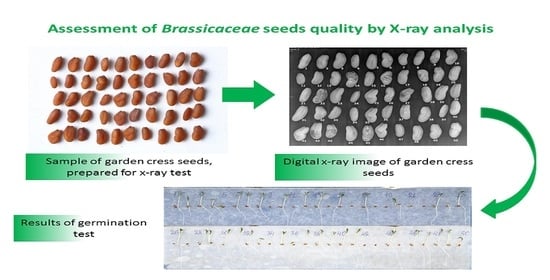
Assessment of Brassicaceae Seeds Quality by X-ray Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Inspection of Brassica oleracea L., var. Capitata Seeds
3.2. Inspection of Raphanus sativus L., var. Radicula Seeds
3.3. Inspection of Lepidium sativum L., Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludilov, V.A.; Alekseev, Y.B.; Timina, O.O. Practical Seed Production of Vegetable Crops with the Basics of Seed Science; Ludilov, V.A., Alekseev, Y.B., Eds.; Scientific Publishing House KMMC: Moscow, Russia, 2011; p. 220. (In Russian) [Google Scholar]
- Bukharov, A.F. Heterogeneity of seeds: Theory and practice (review). Veg. Russ. 2020, 2, 23–31. (In Russian) [Google Scholar]
- Probert, R.J.; Hay, F. Keeping Seeds Alive/Seed Technology and Its Biological Basis; Black, M., Bewley, D., Eds.; Sheffield Academic Press: Sheffield, UK, 2000; pp. 184–225. [Google Scholar]
- Litvinov, S.S.; Razin, A.F.; Mescheryakova, R.A.; Lebedeva, N.N.; Razin, A.A. Safety of vegetable products. Potatoes Veg. 2017, 4, 22–23. (In Russian) [Google Scholar]
- Monakhos, S.G.; Monakhos, G.F. Selection of white cabbage (Brassica oleracea L.) for resistance to keel. Rep. Timiryazev Agric. Acad. 2016, 288, 473–477. (In Russian) [Google Scholar]
- ISO6639/4 -87. Cereals and legumes. In Determination of Hidden Insect Infestations. Part. 4. Accelerated Methods; Standards Publishers: Moskow, Russia, 1987. (In Russian) [Google Scholar]
- GOST 28666.4-90. Cereals and legumes. In Determination of Latent Insect Infestation. Part. 4. Accelerated Methods; Standards Publishers: Moskow, Russia, 1990. (In Russian) [Google Scholar]
- GOST R 59603-2021. Agricultural Seeds. Methods of Digital X-ray; Standartinform: Moscow, Russia, 2021; p. 16. (In Russian) [Google Scholar]
- Musaev, F.B.; Antoshkina, M.S.; Arkhipov, M.V.; Velikanov, L.P.; Gusakova, L.P.; Bessonov, V.B.; Gryaznov, A.Y.; Zhamova, K.K.; Kosov, V.O.; Potrakhov, E.N.; et al. X-ray Quality Analysis of Seeds of Vegetable Crops/Methodological Guidelines; Publishing house of St. Petersburg State Electrotechnical University “LETI”: Moscow, Russia, 2015; p. 42. (In Russian) [Google Scholar]
- Musaev, F.B.; Potrakhov, N.N.; Beletskiy, S.L. BriefAtlas of Radiographic Traits of Seeds of Vegetable Crops; Federal Scientific Center for Vegetable Growing: Moscow, Russia, 2017; p. 40. (In Russian) [Google Scholar]
- Musaev, F.B.; Soldatenko, A.V.; Krivenkov, L.V.; Bukharov, A.F.; Beletskiy, S.L.; Kezimana, P. Assessment of vegetable seeds quality by micro-focus X-ray analysis. Res. Crops 2020, 21, 604–610. [Google Scholar] [CrossRef]
- Gomes-Junior, F.G.; Chiquito, A.A.; Marcos-Filho, J. Semi-automated assessment of the embryonic area of cucumber seeds and its relationship to germination and seedling length. J. Seed Sci. 2013, 35, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.R.; Yasmin, J.; Park, E.; Kim, G.; Kim, M.S.; Wakholi, C.; Mo, C.; Cho, B.K. Classification of watermelon seeds using morphological patterns of X-ray imaging: A comparison of conventional machine learning and deep learning. Sensors 2020, 20, 6753. [Google Scholar] [CrossRef] [PubMed]
- Van der Burg, W.J.; Aartse, J.W.; van Zwol, R.A.; Bino, R.J. Predicting tomato seedling morphology by X-ray analysis of seeds. J. Amer. Soc. Hort. Sci. 1994, 119, 258–263. [Google Scholar] [CrossRef]
- Marcos-Filho, J.; Gomes-Junior, F.G.; Bernett, M.A.; Wells, A.A.; Stieve, S. Using tomato analyzer software to determine embryo size in X-rayed seeds. Rev. Bras. Sementes 2010, 32, 146–153. [Google Scholar] [CrossRef]
- Dell’Aquila, A. Pepper seed germination assessed by combined X-radiography and computer-aided imaging analysis. J. Biologia. Plantarum. 2007, 51, 777–781. [Google Scholar] [CrossRef]
- Abud, H.F.; Cicero, S.M.; Gomes Junior, F.G. Radiographic images and relationship of the internal morphology and physiological potential of broccoli seeds. Acta Scientiarum. Agron. 2018, 40, e34950. [Google Scholar] [CrossRef]
- Bruggink, H.; van Duijn, B. X-Ray based seed image analysis. Seed Test. Int. 2017, 153, 45–50. [Google Scholar]
- Arkhipov, M.V.; Alekseeva, D.I.; Batygin, N.F.; Velikanov, L.P.; Gusakova, L.P.; Derunov, I.V.; Zheludkov, A.G.; Nikolenko, V.F.; Nikitina, L.I.; Savin, V.N.; et al. Method of Radiography in Agriculture and Crop Production; Manual; Publishing House of Russian Academy of Agricultural Sciences: Moskov, Russia, 2001; p. 93. (In Russian) [Google Scholar]
- Lychkovskaya, I.Y.; Slukin, A.S. Distribution of phytophage insects on oil-bearing cabbage crops under conditions of dry vegetation period. Rep. Russ. Acad. Agric. Sci. 2013, 1, 28–30. (In Russian) [Google Scholar]
- Hokkanen, H.M.T. Biological and agrotechnical control of the rape blossom beetle Meligethes aeneus (Coleoptera, Nitidulidae). Actaentomol. Fenn. 1989, 53, 25–29. [Google Scholar]
- Arkhipov, M.V.; Priyatkin, N.S.; Gusakova, L.P.; Potrakhov, N.N.; Gryaznov, A.Y.; Bessonov, V.B.; Obodovsky, A.V.; Staroverov, N.E. X-Ray Computer Methods for Studying the Structural Integrity of Seeds and Their Importance in Modern Seed Science. Tech. Phys. 2019, 64, 582–592. [Google Scholar] [CrossRef]
- Tkachenko, K.G.; Staroverov, N.E.; Gryaznov, A.Y. Radiographic study of fruit and seed quality. Hortus Bot. 2018, 13, 52–66. (In Russian) [Google Scholar] [CrossRef]
- Silva, V.N.; Cicero, S.M.; Bennett, M. Relationship between eggplant seed morphology and germination. Rev. Bras. Sementes 2012, 34, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Orru, M.; Grillo, O.; Venora, G.; Bacchetta, G. Computer vision as a method complementary to molecular analysis: Grapevine cultivar seeds case study. Comptes. Rendus. Biol. 2012, 335, 602–615. [Google Scholar] [CrossRef]
- Lo Bianco, M.; Grillo, O.; Canadas, E.; Venora, G.; Bacchetta, G. Inter- and intraspecic diversity in Cistus L. (Cistaceae) seeds, analysed with computer vision techniques. Plant Biol. 2017, 19, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Loddo, A.; Loddo, M.; Di Ruberto, C. A Novel deep learning based approach for seed image classification and retrieval. Comput. Electron. Agric. 2021, 187, 106269. [Google Scholar] [CrossRef]
- Severiano, R.L.; Pinheiro, P.R.; Gomes Junior, F.G.; de Medeiros, A.D.; Pereira, M.D. X-ray test on passion fruit seeds submitted to different aryl removal methods. Comun. Sci. 2018, 9, 356–362. [Google Scholar] [CrossRef] [Green Version]
- De Medeiros, A.D.; Zavala-León, M.J.; de Oliveira Araújo, J.; Pereira, M.D.; dos Santos, D.C.F.; Silva, D.L.J. Relationship between internal morphology and physiological quality of Leucaena leucocephala seeds using image analysis. Rev. Árvore 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Derunov, I.V. Radiographic Study of Seeds of Various Agricultural Crops and Products of Their Processing: Abstract of Ph; Candidate of Biological Sciences ARI: Saint Petersburg, Russia, 2004; p. 22. (In Russian) [Google Scholar]
- Gusakova, L.P. X-Ray and Cytophotometric Analysis of the Viability of Agricultural Crop Seeds: Extended Abstract of Cand; Science Dissertation: Saint-Petersburg, Russia, 1997; p. 20. (In Russian) [Google Scholar]















| Sample | Variety | Maintainer | Production Area |
|---|---|---|---|
| Brassica oleracea L., var. capitata | Amager 611 | FSVC | Derbent, Russia |
| Brassica oleracea L., var. capitata | Belorusskaya 455 | FSVC | Moscow Region, Russia |
| Brassica oleracea L., var. capitata | Moskovskaya pozdnyaya 15 | FSVC | Moscow Region, Russia |
| Brassica oleracea L., var. capitata | Podarok 2500 | FSVC | Derbent, Russia |
| Brassica oleracea L., var. capitata | F1 Malakhit | RSAA | Italy |
| Brassica oleracea L., var. capitata | F1 Transfer | RSAA | Italy |
| Brassica oleracea L., var. capitata | F1 Valentina | RSAA | Italy |
| Brassica oleracea L., var. capitata | F1 Valentina | RSAA | Australia |
| Raphanus sativus L., var. radicula | Ariya | FSVC | Moscow Region, Russia |
| Raphanus sativus L., var. radicula | Pink-red with a white tip | FSVC | Moscow Region, Russia |
| Raphanus sativus L., var. radicula | Niger, population I3 | FSVC | Moscow Region, Russia |
| Raphanus sativus L., var. radicula | Inbred line I4 | FSVC | Moscow Region, Russia |
| Lepidium sativum L., | Prestige | FSVC | Moscow Region, Russia |
| Lepidium sativum L., | Flagman | FSVC | Moscow Region, Russia |
| Lepidium sativum L., | Mechta Derbenta | RSAA | Derbent, Russia |
| Parameters | Varieties | |||
|---|---|---|---|---|
| Amager | Belorusskaya | Moskovskaya Pozdnyaya | Podarok | |
| Trait according to the results of radiographic analysis, % | ||||
| Normal | 45 | 41 | 42 | 56 |
| Empty | 0 | 0 | 14 | 0 |
| Germ partitioning of germ parts with soft partitioning | 11 | 8 | 8 | 9 |
| Patterned | 17 | 16 | 5 | 22 |
| Angulated | 13 | 23 | 22 | 10 |
| With irregular shading | 14 | 12 | 9 | 3 |
| Germination, % | ||||
| Germinated | 54 | 52 | 48 | 62 |
| Sprouting | 16 | 14 | 11 | 18 |
| Not germinated | 30 | 34 | 39 | 20 |
| Parameter | Seed ID | ||
|---|---|---|---|
| 38 | 39 | 40 | |
| Average Brightness, brightness units | 162 | 184 | 162 |
| Brightness Deviation, brightness units | 39 | 42 | 45 |
| Parameter | Seed ID | ||
|---|---|---|---|
| 45 | 46 | 47 | |
| Average Brightness, brightness units | 165 | 142 | 193 |
| Brightness Deviation, brightness units | 42 | 35 | 47 |
| Parameter | Seed ID | ||
|---|---|---|---|
| 14 | 18 | 16 | |
| Roundness, nondimensional value | 0.734 | 0.647 | 0.890 |
| Circle factor, nondimensional value | 0.927 | 0.907 | 0.991 |
| Parameters of X-ray Pattern of Seed | Seed ID | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | |
 | ||||||||||
| Average Brightness, Brightness Units | 184 | 182 | 175 | 185 | 168 | 184 | 184 | 188 | 187 | 184 |
| Brightness Standard Deviation, Brightness Units | 25 | 33 | 23 | 26 | 27 | 28 | 28 | 26 | 26 | 30 |
| Replications | X-ray Analysis, % | Laboratory Germination, % | ||
|---|---|---|---|---|
| Normal | Insects Damaged | Irregular Shadings | ||
| 1 | 76 | 14 | 10 | 80 |
| 2 | 72 | 20 | 8 | 74 |
| 3 | 64 | 36 | 0 | 72 |
| 4 | 85 | 12 | 3 | 82 |
| Mean | 74.0 | 20.5 | 5.0 | 77.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musaev, F.; Priyatkin, N.; Potrakhov, N.; Beletskiy, S.; Chesnokov, Y. Assessment of Brassicaceae Seeds Quality by X-ray Analysis. Horticulturae 2022, 8, 29. https://doi.org/10.3390/horticulturae8010029
Musaev F, Priyatkin N, Potrakhov N, Beletskiy S, Chesnokov Y. Assessment of Brassicaceae Seeds Quality by X-ray Analysis. Horticulturae. 2022; 8(1):29. https://doi.org/10.3390/horticulturae8010029
Chicago/Turabian StyleMusaev, Farhad, Nikolay Priyatkin, Nikolay Potrakhov, Sergey Beletskiy, and Yuri Chesnokov. 2022. "Assessment of Brassicaceae Seeds Quality by X-ray Analysis" Horticulturae 8, no. 1: 29. https://doi.org/10.3390/horticulturae8010029
APA StyleMusaev, F., Priyatkin, N., Potrakhov, N., Beletskiy, S., & Chesnokov, Y. (2022). Assessment of Brassicaceae Seeds Quality by X-ray Analysis. Horticulturae, 8(1), 29. https://doi.org/10.3390/horticulturae8010029