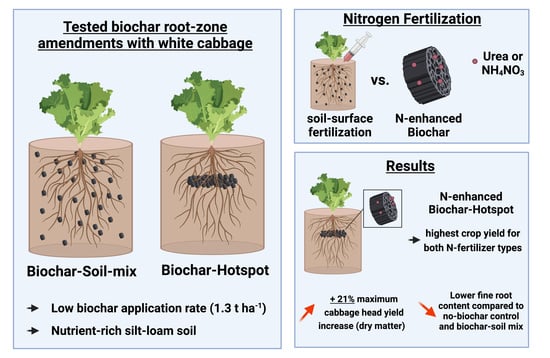
Root-Zone Amendments of Biochar-Based Fertilizers: Yield Increases of White Cabbage in Temperate Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Origin and Further Processing
2.2. Combining Biochar and N Fertilizer
2.3. Greenhouse Trial
2.4. Aboveground Biomass Harvest and Data Collection
2.5. Belowground Biomass Analysis
2.6. Vitamin-C Quantification
2.7. Data Analysis
3. Results and Discussion
3.1. Aboveground Biomass Yields
3.2. Belowground Biomass Yields and Root Architecture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.; Bucheli, T. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in Agriculture—A Systematic Review of 26 Global Meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Kammann, C.; Ippolito, J.; Hagemann, N.; Borchard, N.; Cayuela, M.L.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Jeffery, S.; Kern, J.; Novak, J.; et al. Biochar as a Tool to Reduce the Agricultural Greenhouse-Gas Burden—Knowns, Unknowns and Future Research Needs. J. Environ. Eng. Landsc. Manag. 2017, 25, 114–139. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar Effects on Crop Yields with and without Fertilizer: A Meta-analysis of Field Studies Using Separate Controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Bai, S.H.; Omidvar, N.; Gallart, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.B.; Singh, K.; Zhou, G.; Muqadass, B.; Xu, C.-Y.; et al. Combined Effects of Biochar and Fertilizer Applications on Yield: A Review and Meta-Analysis. Sci. Total Environ. 2022, 808, 152073. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Pandit, B.H.; Cornelissen, G.; Kammann, C.I. Biochar-Based Fertilization with Liquid Nutrient Enrichment: 21 Field Trials Covering 13 Crop Species in Nepal: Biochar-Based Fertilization. Land Degrad. Develop. 2017, 28, 2324–2342. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Lehmann, J.; Carneiro, J.S.D.S.; Camps-Arbestain, M. Biochar-Based Fertilizer Effects on Crop Productivity: A Meta-Analysis. Plant. Soil 2022, 472, 45–58. [Google Scholar] [CrossRef]
- Gwenzi, W.; Nyambishi, T.J.; Chaukura, N.; Mapope, N. Synthesis and Nutrient Release Patterns of a Biochar-Based N–P–K Slow-Release Fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 405–414. [Google Scholar] [CrossRef]
- Liu, X.; Liao, J.; Song, H.; Yang, Y.; Guan, C.; Zhang, Z. A Biochar-Based Route for Environmentally Friendly Controlled Release of Nitrogen: Urea-Loaded Biochar and Bentonite Composite. Sci. Rep. 2019, 9, 9548. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar Bound Urea Boosts Plant Growth and Reduces Nitrogen Leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef]
- Shi, W.; Bian, R.; Li, L.; Lian, W.; Liu, X.; Zheng, J.; Cheng, K.; Zhang, X.; Drosos, M.; Joseph, S.; et al. Assessing the Impacts of Biochar-blended Urea on Nitrogen Use Efficiency and Soil Retention in Wheat Production. GCB Bioenergy 2022, 14, 65–83. [Google Scholar] [CrossRef]
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.; Rutherford, D.; Sparrevik, M.; Hale, S.; Obia, A.; Mulder, J. Biochar Effect on Maize Yield and Soil Characteristics in Five Conservation Farming Sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, P.; Krull, E.; Butler, G.; Herbert, A.; Solaiman, Z. Effect of Banded Biochar on Dryland Wheat Production and Fertiliser Use in South-Western Australia: An Agronomic and Economic Perspective. Soil Res. 2010, 48, 531. [Google Scholar] [CrossRef]
- Schmidt, H.; Pandit, B.; Martinsen, V.; Cornelissen, G.; Conte, P.; Kammann, C. Fourfold Increase in Pumpkin Yield in Response to Low-Dosage Root Zone Application of Urine-Enhanced Biochar to a Fertile Tropical Soil. Agriculture 2015, 5, 723–741. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L. Participation of Urea-N Absorbed on Biochar Granules among Soil and Tobacco Plant (Nicotiana tabacum, L.) and Its Potential Environmental Impact. Agric. Ecosyst. Environ. 2021, 313, 107371. [Google Scholar] [CrossRef]
- Altaf, F.; Gul, S.; Chandio, T.A.; Rehman, G.B.; Kakar, A.-R.; Khan, N.; Shaheen, U.; Shahwani, M.N.; Ajmal, M.; Manzoor, M. Influence of Biochar Based Organic Fertilizers on Growth and Concentration of Heavy Metals in Tomato and Lettuce in Chromite Mine Tailing Contaminated Soil. Sarhad J. Agric. 2021, 37, 315–324. [Google Scholar] [CrossRef]
- Colombi, T.; Kirchgessner, N.; Le Marié, C.A.; York, L.M.; Lynch, J.P.; Hund, A. Next Generation Shovelomics: Set up a Tent and REST. Plant. Soil 2015, 388, 1–20. [Google Scholar] [CrossRef]
- Abiven, S.; Hund, A.; Martinsen, V.; Cornelissen, G. Biochar Amendment Increases Maize Root Surface Areas and Branching: A Shovelomics Study in Zambia. Plant. Soil 2015, 395, 45–55. [Google Scholar] [CrossRef] [Green Version]
- EBC (2012–2022) “European Biochar Certificate—Guidelines for a Sustainable Production of Biochar.” European Biochar Foundation (EBC), Arbaz, Switzerland. Available online: http://european-biochar.org (accessed on 10 January 2022).
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of Time and Temperature on Vitamin C Stability in Horticultural Extracts. UHPLC-PDA vs. Iodometric Titration as Analytical Methods. LWT-Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- Piepho, H.P.; Edmondson, R.N. A Tutorial on the Statistical Analysis of Factorial Experiments with Qualitative and Quantitative Treatment Factor Levels. J. Agro. Crop Sci. 2018, 204, 429–455. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Upadhyay, A.K.; Bahadur, A.; Singh, B.; Singh, K.P.; Rai, M. Antioxidant Phytochemicals in Cabbage (Brassica oleracea, L. Var. Capitata). Sci. Hortic. 2006, 108, 233–237. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Mena, P.; García-Viguera, C.; Moreno, D.A. Brassica Foods as a Dietary Source of Vitamin C: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef]
- Mazid Miah, M.A.; Gaihre, Y.K.; Hunter, G.; Singh, U.; Hossain, S.A. Fertilizer Deep Placement Increases Rice Production: Evidence from Farmers’ Fields in Southern Bangladesh. Agron. J. 2016, 108, 805–812. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhou, J.; Chen, Z.; Lu, D.; Zhu, D.; Deng, P. Effect of Nitrogen Root Zone Fertilization on Rice Yield, Uptake and Utilization of Macronutrient in Lower Reaches of Yangtze River, China. Paddy Water Environ. 2017, 15, 625–638. [Google Scholar] [CrossRef]
- Jiang, C.; Lu, D.; Zu, C.; Zhou, J.; Wang, H. Root-Zone Fertilization Improves Crop Yields and Minimizes Nitrogen Loss in Summer Maize in China. Sci. Rep. 2018, 8, 15139. [Google Scholar] [CrossRef] [Green Version]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant Growth Improvement Mediated by Nitrate Capture in Co-Composted Biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Bavarian State Agency for Agriculture Contribution Margins and Calculation Data—White Cabbage (Industrial Product). Available online: https://www.stmelf.bayern.de/idb/weisskohl.html (accessed on 9 February 2022).
- German Federal Statistical Office Farms, Areas under Cultivation, Yields and Harvest Quantities of Vegetables. Available online: https://www.destatis.de/DE/Themen/Branchen-Unternehmen/Landwirtschaft-Forstwirtschaft-Fischerei/Obst-Gemuese-Gartenbau/Tabellen/betriebe-anbau-erntemenge-gemuese.html#fussnote-A-123138 (accessed on 9 February 2022).
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of Biochar Application on Root Traits: A Meta-Analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]





| Treatment ID | Nitrogen Fertilizer | Type of Biochar Root-Zone Amendment | Nitrogen Fertilization Method |
|---|---|---|---|
| 1 | none | none | n.a. |
| 2 | NH4NO3 | none | soil-surface fertilization |
| 3 | NH4NO3 | Hotspot | N-enhanced biochar |
| 4 | NH4NO3 | Hotspot | soil-surface fertilization |
| 5 | NH4NO3 | Soil-Mix | N-enhanced biochar |
| 6 | NH4NO3 | Soil-Mix | soil-surface fertilization |
| 7 | Urea | none | soil-surface fertilization |
| 8 | Urea | Hotspot | N-enhanced biochar |
| 9 | Urea | Hotspot | soil-surface fertilization |
| 10 | Urea | Soil-Mix | N-enhanced biochar |
| 11 a | Urea | Soil-Mix | soil-surface fertilization |
| Treatment | Aboveground Biomass (FM) | Cabbage Heads (FM) | Aboveground Biomass (DM) | Cabbage-Head Ratio (DM) | Cabbage-Head Volume | SPAD | Vitamin C Content in Head Biomass | Dry Root Biomass |
|---|---|---|---|---|---|---|---|---|
| g | g | g | wt% | cm3 | (mg(100 g)−1) | g | ||
| 1: No N-Fert., no BC | 106.1 ± 6.9 b | n.a. | 17.5 ± 1.5 b | n.a. | n.a. | 48.0 ± 1.7 a | n.a. | 1.4 ± 0.1 a |
| NH4NO3-Fertilizer | ||||||||
| 2: no BC-Control | 430.9 ± 34.5 a | 206.8 ± 32.5 a | 29.4 ± 0.8 a | 47.0 ± 4.0 b | 2270.5 ± 313.1 a | 53.6 ± 1.9 a | 7.1 ± 0.2 a | 1.4 ± 0.3 a |
| 3: BC-Hotspot, N-enhanced BC | 465.5 ± 5.4 a | 271.7 ± 7.0 a | 28.5 ± 0.9 a | 58.4 ± 1.3 a | 2874.1 ± 133.2 a | 54.0 ± 1.7 a | 7.4 ± 0.7 a | 1.3 ± 0.1 a |
| 4: BC-Hotspot, pure BC | 470.9 ± 15.3 a | 263.6 ± 8.1 a | 30.8 ± 0.7 a | 56.0 ± 0.9 ab | 2803.5 ± 134.3 a | 54.8 ± 1.7 a | 8.5 ± 0.3 a | 1.5 ± 0.1 a |
| 5: BC-mixed, N-enhanced BC | 436.9 ± 9.5 a | 227.5 ± 7.0 a | 29.2 ± 0.5 a | 52.1 ± 1.1 ab | 2420.3 ± 81.5 a | 54.3 ± 1.7 a | 7.0 ± 0.1 a | 1.6 ± 0.2 a |
| 6: BC-mixed, pure BC | 465.1 ± 21.3 a | 246.1 ± 12.6 a | 30.9 ± 1.5 a | 53.1 ± 2.3 ab | 2464 ± 142.2 a | 53.9 ± 1.7 a | 8.1 ± 0.9 a | 1.6 ± 0.2 a |
| Urea-Fertilizer | ||||||||
| 7: no BC-Control | 452.1 ± 10.5 a | 236.8 ± 5.8 a | 29.2 ± 0.6 a | 52.4 ± 1.3 ab | 2401.1 ± 106.5 a | 54.5 ± 2.8 a | 7.8 ± 0.4 a | 1.4 ± 0.1 a |
| 8: BC-Hotspot, N-enhanced BC | 447.5 ± 4.6 a | 258.9 ± 8.7 a | 30.04 ± 0.9 a | 57.8 ± 1.4 a | 2691.8 ± 88.8 a | 56.9 ± 2.8 a | 8.0 ± 0.2 a | 1.5 ± 0.1 a |
| 9: BC-Hotspot, pure BC | 433.7 ± 21.6 a | 234.1 ± 21.5 a | 27.9 ± 1.2 a | 53.8 ± 3.5 ab | 2557.3 ± 296.6 a | 54.0 ± 1.2 a | 7.7 ± 0.5 a | 1.4 ± 0.1 a |
| 10: BC-mixed, N-enhanced BC | 439.5 ± 7.7 a | 259.3 ± 7.9 a | 27.9 ± 1.1 a | 59.0 ± 1.1 a | 2615.0 ± 195.3 a | 56.7 ± 2.4 a | 7.2 ± 0.2 a | 1.5 ± 0.1 a |
| p-Value (ANOVA) | <0.0001 | 0.06 | <0.0001 | 0.01 | 0.33 | 0.18 | 0.38 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafmüller, J.; Schmidt, H.-P.; Kray, D.; Hagemann, N. Root-Zone Amendments of Biochar-Based Fertilizers: Yield Increases of White Cabbage in Temperate Climate. Horticulturae 2022, 8, 307. https://doi.org/10.3390/horticulturae8040307
Grafmüller J, Schmidt H-P, Kray D, Hagemann N. Root-Zone Amendments of Biochar-Based Fertilizers: Yield Increases of White Cabbage in Temperate Climate. Horticulturae. 2022; 8(4):307. https://doi.org/10.3390/horticulturae8040307
Chicago/Turabian StyleGrafmüller, Jannis, Hans-Peter Schmidt, Daniel Kray, and Nikolas Hagemann. 2022. "Root-Zone Amendments of Biochar-Based Fertilizers: Yield Increases of White Cabbage in Temperate Climate" Horticulturae 8, no. 4: 307. https://doi.org/10.3390/horticulturae8040307
APA StyleGrafmüller, J., Schmidt, H. -P., Kray, D., & Hagemann, N. (2022). Root-Zone Amendments of Biochar-Based Fertilizers: Yield Increases of White Cabbage in Temperate Climate. Horticulturae, 8(4), 307. https://doi.org/10.3390/horticulturae8040307