Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means
Abstract
:1. Introduction
2. Plant Polyphenols as Antimicrobials
2.1. Flavonoids
2.2. Non-Flavonoids
2.3. Extraction of Polyphenols from Plant Products
3. Antimicrobial Activity and Structural Relationship of Plant-Derived Polyphenols
3.1. Position of Functional Group
3.1.1. Chalcones
3.1.2. Flavanes and Flavanols
3.1.3. Flavonols
3.1.4. Flavones
3.2. Number of Functional Groups Attached
3.3. Presence of C2=C3 Double Bond
3.4. Type of Functional Group
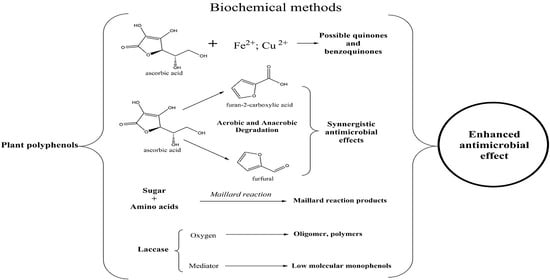
4. Enhancement of Antimicrobial Activity of Plant Derived Polyphenols by Biochemical Methods
4.1. Enhancement Using Ascorbic Acid and Transition Metals
4.2. Enhancement Using Degradation Products of Ascorbic Acid in an Ethanolic Solution
4.3. Enhancement Using Maillard Reaction Products
4.4. Enhancement Using Laccase–Mediator System
4.5. Enhancement Using Peroxidase Enzyme
4.6. Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbosa-Pereira, L.; Bilbao, A.; Vilches, P.; Angulo, I.; LLuis, J.; Fite, B.; Paseiro-Losada, P.; Cruz, J.M. Brewery waste as a potential source of phenolic compounds: Optimisation of the extraction process and evaluation of antioxidant and antimicrobial activities. Food Chem. 2014, 145, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Schuenzel, K.M.; Harrison, M.A. Microbial antagonists of foodborne pathogens on fresh, minimally processed vegetables. J. Food Prot. 2002, 65, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.A.; Lucera, A.; Costa, C.; Conte, A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 103–115. [Google Scholar]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bor, T.; Aljaloud, S.O.; Gyawali, R.; Ibrahim, S.A. Chapter 26—Antimicrobials from herbs, spices, and plants. In Fruits, Vegetables, and Herbs; Watson, R.R., Preedy, V.R., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.A.; Desai, S.D.; Singh, J. A Review on Plant Antimicrobials of Past Decade. Curr. Top. Med. Chem. 2018, 18, 812–833. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Hashemi, S.M.B.; Es, I.; Fracassetti, D.; Limbo, S. Efficacy of Antimicrobial Agents for Food Contact Applications: Biological Activity, Incorporation into Packaging, and Assessment Methods: A Review. J. Food Prot. 2018, 81, 1142–1156. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol-protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhou, T.; Tsao, R. Antimicrobials from Plants—Food Preservation and Shelf-Life Extension; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 645–658. [Google Scholar]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; Vazquez Gonzalez, Y.; Ragazzo-Sánchez, J.A. Application of essential oils and polyphenols as natural antimicrobial agents in postharvest treatments: Advances and challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Hygreeva, D.; Pandey, M.C.; Radhakrishna, K. Potential applications of plant based derivatives as fat replacers, antioxidants and antimicrobials in fresh and processed meat products. Meat Sci. 2014, 98, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Rio, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639–1648. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med. 2016, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review. Molecules 2022, 27, 1484. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720–110442. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Alvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Herald, P.J.; Davidson, P.M. Antibacterial Activity of Selected Hydroxycinnamic Acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Chipley, J.R.; Uraih, N. Inhibition of Aspergillus growth and aflatoxin release by derivatives of benzoic acid. Appl. Environ. Microbiol. 1980, 40, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Ulate-Rodriguez, J.; Schafer, H.W.; Zottola, E.A.; Davidson, P.M. Inhibition of Listeria monocytogenes, Escherichia coli O157:H7, and Micrococcus luteus by Linear Furanocoumarins in Culture Media. J. Food Prot. 1997, 60, 1046–1049. [Google Scholar] [CrossRef]
- Dogasaki, C.; Shindo, T.; Furuhata, K.; Fukuyama, M. Identification of chemical structure of antibacterial components against Legionella pneumophila in a coffee beverage. Yakugaku Zasshi 2002, 122, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.A.; Salameh, M.M.; Phetsomphou, S.; Yang, H.; Seo, C.W. Application of caffeine, 1,3,7-trimethylxanthine, to control Escherichia coli O157:H7. Food Chem. 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Kim, S.; Fung, D.Y. Antibacterial effect of crude water-soluble arrowroot (Puerariae radix) tea extracts on food-borne pathogens in liquid medium. Lett. Appl. Microbiol. 2004, 39, 319–325. [Google Scholar] [CrossRef]
- Tadakatsu, S.; Wei-Hua, Z.; Zhi-Qing, H. Mechanism of Action and Potential for Use of Tea Catechin as an Antiinfective Agent. Anti-Infect. Agents Med. Chem. 2007, 6, 57–62. [Google Scholar]
- Lee, Y.L.; Cesario, T.; Wang, Y.; Shanbrom, E.; Thrupp, L. Antibacterial activity of vegetables and juices. Nutrition 2003, 19, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial Properties and Major Bioactive Components of Cinnamon Stick (Cinnamomum burmannii): Activity against Foodborne Pathogenic Bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef] [PubMed]
- Dassoff, E.S.; Li, Y.O. Mechanisms and effects of ultrasound-assisted supercritical CO2 extraction. Trends Food Sci. Technol. 2019, 86, 492–501. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Wu, N.-N.; Li, H.-H.; Tan, B.; Zhang, M.; Xiao, Z.-G.; Tian, X.-H.; Zhai, X.-T.; Liu, M.; Liu, Y.-X.; Wang, L.-P. Free and bound phenolic profiles of the bran from different rice varieties and their antioxidant activity and inhibitory effects on ɑ-amylose and ɑ-glucosidase. J. Cereal Sci. 2018, 82, 206–212. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547–100556. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. O-Glycoside quercetin derivatives: Biological activities, mechanisms of action, and structure–activity relationship for drug design, a review. Phytother. Res. 2022, 36, 778–807. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure–activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Toth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Omosa, L.K.; Midiwo, J.O.; Mbaveng, A.T.; Tankeo, S.B.; Seukep, J.A.; Voukeng, I.K.; Dzotam, J.K.; Isemeki, J.; Derese, S.; Omolle, R.A.; et al. Antibacterial activities and structure-activity relationships of a panel of 48 compounds from Kenyan plants against multidrug resistant phenotypes. Springerplus 2016, 5, 901–916. [Google Scholar] [CrossRef] [Green Version]
- Mbaveng, A.T.; Ngameni, B.; Kuete, V.; Simo, I.K.; Ambassa, P.; Roy, R.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 2008, 116, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Oh, I.; Yang, W.Y.; Chung, S.C.; Kim, T.Y.; Oh, K.B.; Shin, J. In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch. Pharm. Res. 2011, 34, 217–222. [Google Scholar] [CrossRef]
- Osorio, M.; Carvajal, M.; Vergara, A.; Butassi, E.; Zacchino, S.; Mascayano, C.; Montoya, M.; Mejías, S.; Martín, M.C.-S.; Vásquez-Martínez, Y. Prenylated Flavonoids with Potential Antimicrobial Activity: Synthesis, Biological Activity, and In Silico Study. Int. J. Mol. Sci. 2021, 22, 5472. [Google Scholar] [CrossRef]
- Smejkal, K.; Chudik, S.; Kloucek, P.; Marek, R.; Cvacka, J.; Urbanova, M.; Julinek, O.; Kokoska, L.; Slapetova, T.; Holubova, P.; et al. Antibacterial C-geranylflavonoids from Paulownia tomentosa Fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Bitchagno, G.T.; Sama Fonkeng, L.; Kopa, T.K.; Tala, M.F.; Kamdem Wabo, H.; Tume, C.B.; Tane, P.; Kuiate, J.R. Antibacterial activity of ethanolic extract and compounds from fruits of Tectona grandis (Verbenaceae). BMC Complement. Altern. Med. 2015, 15, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Bitchagno, G.T.M.; Tankeo, S.B.; Tsopmo, A.; Mpetga, J.D.S.; Tchinda, A.T.; Fobofou, S.A.T.; Wessjohann, L.A.; Kuete, V.; Tane, P. Lemairones A and B: Two new antibacterial tetraflavonoids from the leaves of Zanthoxylum lemairei (Rutaceae). Phytochem. Lett. 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Tebou, P.L.F.; Tamokou, J.D.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Kuiate, J.R.; Bag, P.K. Flavonoids from Maytenus buchananii as potential cholera chemotherapeutic agents. S. Afr. J. Bot. 2017, 109, 58–65. [Google Scholar] [CrossRef]
- Babajide, O.J.; Babajide, O.O.; Daramola, A.O.; Mabusela, W.T. Flavonols and an oxychromonol from Piliostigma reticulatum. Phytochemistry 2008, 69, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Novak, Z.; Chlebek, J.; Opletal, L.; Jiros, P.; Macakova, K.; Kunes, J.; Cahlikova, L. Corylucinine, a new alkaloid from Corydalis cava (Fumariaceae), and its cholinesterase activity. Nat. Prod. Commun. 2012, 7, 859–860. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Zhou, L.; Zhou, Y.; Chen, Y.; Sui, P.; Wang, J.; Wang, M. Antimicrobial flavonoids from the twigs of Populus nigra x Populus deltoides. Nat. Prod. Res. 2012, 26, 307–313. [Google Scholar] [CrossRef]
- Alcaraz, L.E.; Blanco, S.E.; Puig, O.N.; Tomas, F.; Ferretti, F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Hummelova, J.; Rondevaldova, J.; Balastikova, A.; Lapcik, O.; Kokoska, L. The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett. Appl. Microbiol. 2015, 60, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H. Isoflavanones from the heartwood of Swartzia polyphylla and their antibacterial activity against cariogenic bacteria. Chem. Pharm. Bull. 1992, 40, 2970–2974. [Google Scholar] [CrossRef] [Green Version]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Res. Int. 2018, 2018, 7413504–7413513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial Activity of Polyphenols: Structure-Activity Relationship and Influence of Hyperglycemic Condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhou, J.; Li, D.; Shang, C.; Peng, L.; Pan, S. The structure-antifungal activity relationship of 5,7-dihydroxyflavonoids against Penicillium italicum. Food Chem. 2017, 224, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-L.; Wang, H.-D.; Lee, S.M.; Wang, Y.-T.; Du, G.-H. Structure—Activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.; Dzuvor, C.K.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Kuete, V.; Alibert-Franco, S.; Eyong, K.O.; Ngameni, B.; Folefoc, G.N.; Nguemeving, J.R.; Tangmouo, J.G.; Fotso, G.W.; Komguem, J.; Ouahouo, B.M.; et al. Antibacterial activity of some natural products against bacteria expressing a multidrug-resistant phenotype. Int. J. Antimicrob. Agents 2011, 37, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-tuning of the hydrophobicity of caffeic acid: Studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef] [Green Version]
- Kardum, N.; Glibetic, M. Polyphenols and their interactions with other dietary compounds: Implications for human health. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 84, pp. 103–144. [Google Scholar]
- Weissberger, A.; LuValle, J.E. Oxidation Processes. XVII.1 The Autoxidation of Ascorbic Acid in the Presence of Copper. J. Am. Chem. Soc. 1944, 66, 700–705. [Google Scholar] [CrossRef]
- Shtamm, E.V.; Purmal, A.P.; Skurlatov, Y.I. The role of hydrogen peroxide in natural aquatic media. Int. J. Chem. Kin. 1991, 5, 1228–1248. [Google Scholar] [CrossRef]
- Baker, W.L.; Goode, J.; Cooper, L. Estimation of Hydrogen Peroxide Formed and Residual Ascorbate in the Copper Catalysed Oxidation Reaction of Ascorbate at pH 7. Mikrochim. Acta 1992, 106, 143–152. [Google Scholar] [CrossRef]
- Jansson, P.J.; Asplund, K.U.; Makela, J.C.; Lindqvist, C.; Nordstrom, T. Vitamin C (ascorbic acid) induced hydroxyl radical formation in copper contaminated household drinking water: Role of bicarbonate concentration. Free Radic Res. 2003, 37, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Erdem, G.; Oner, C.; Onal, A.M.; Kisakurek, D.; Ogus, A. Free radical mediated interaction of ascorbic acid and ascorbate/Cu(II) with viral and plasmid DNAs. J. Biosci. 1994, 19, 9–17. [Google Scholar] [CrossRef]
- Nappi, A.J.; Emily, V. Hydroxyl radical production by ascorbate and hydrogen peroxide. Neurotox Res. 2012, 2, 343–355. [Google Scholar] [CrossRef]
- Akbıyık, T.; Sönmezoğlu, İ.; Güçlü, K.; Tor, İ.; Apak, R. Protection of Ascorbic Acid from Copper(II)–Catalyzed Oxidative Degradation in the Presence of Fruit Acids: Citric, Oxalic, Tartaric, Malic, Malonic, and Fumaric Acids. Int. J. Food Prop. 2012, 15, 398–411. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G.L. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 44, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Zhou, J.; Heng, D.; Su, X.; Onakpa, M.M.; Bai, Y.; Duan, J.-A.; Che, C.-T.; Bi, H.; Zhao, M. Quinone Derivatives as Promising Anti-Helicobacter pylori Agents from Aerial Parts of Mitracarpus hirtus. J. Nat. Prod. 2022, 85, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Mone, N.S.; Bhagwat, S.A.; Sharma, D.; Chaskar, M.; Patil, R.H.; Zamboni, P.; Nawani, N.N.; Satpute, S.K. Naphthoquinones and Their Derivatives: Emerging Trends in Combating Microbial Pathogens. Coatings 2021, 11, 434. [Google Scholar] [CrossRef]
- Goel, S.; Parihar, P.S.; Meshram, V. Plant-derived quinones as a source of antibacterial and anticancer agents. In Bioactive Natural Products in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 245–279. [Google Scholar]
- Zhang, L.; Zhang, G.; Xu, S.; Song, Y. Recent advances of quinones as a privileged structure in drug discovery. Eur. J. Med. Chem. 2021, 223, 113632–113647. [Google Scholar] [CrossRef]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Hirasawa, T.; Maruyama, Y.; Ishii, Y.; Ito, R.; Saito, K.; Umemara, T.; Nishikawa, A.; Nakazawa, H. Effect of interaction between phenolic compounds and copper ion onantioxidant and pro-oxidant activities. Toxicol. Vitr. 2011, 25, 1320–1327. [Google Scholar] [CrossRef]
- Hoshino, N.; Kimura, T.; Hayakawa, F.; Yamaji, A.; Ando, T. Bactericidal activity of catechin-copper (II) complexes against Staphylococcus aureus compared with Escherichia coli. Lett. Appl. Microbiol. 2000, 31, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.W.; Fielder, M.D.; Kelly, A.F.; El Sankary, W.; Naughton, D.P. Antimicrobial pomegranate rind extracts: Enhancement by Cu(II) and vitamin C combinations against clinical isolates of Pseudomonas aeruginosa. Br. J. Biomed. Sci. 2009, 66, 129–132. [Google Scholar] [CrossRef]
- McCarrell, E.M.; Gould, S.W.; Fielder, M.D.; Kelly, A.F.; El Sankary, W.; Naughton, D.P. Antimicrobial activities of pomegranate rind extracts: Enhancement by addition of metal salts and vitamin C. BMC Complement. Altern. Med. 2008, 8, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Holloway, A.C.; Gould, S.W.; Fielder, M.D.; Naughton, D.P.; Kelly, A.F. Enhancement of antimicrobial activities of whole and sub-fractionated white tea by addition of copper (II) sulphate and vitamin C against Staphylococcus aureus; a mechanistic approach. BMC Complement. Altern. Med. 2011, 11, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Holloway, A.C.; Mueller-Harvey, I.; Gould, S.W.; Fielder, M.D.; Naughton, D.P.; Kelly, A.F. The effect of copper(II), iron(II) sulphate, and vitamin C combinations on the weak antimicrobial activity of (+)-catechin against Staphylococcus aureus and other microbes. Met. Integr. Biometal Sci. 2012, 4, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Srivastava, S.K.; Gaurav, A.; Kumar, A.; Kumar, P.; Yadav, A.S.; Pathania, R.; Navani, N.K. A Combination of Linalool, Vitamin C, and Copper Synergistically Triggers Reactive Oxygen Species and DNA Damage and Inhibits Salmonella enterica subsp. enterica Serovar Typhi and Vibrio fluvialis. Appl. Environ. Microbiol. 2019, 85, 02487–02518. [Google Scholar] [CrossRef] [Green Version]
- Njus, D.; Wigle, M.; Kelley, P.M.; Kipp, B.H.; Schlegel, H.B. Mechanism of ascorbic acid oxidation by cytochrome b(561). Biochemistry 2001, 40, 11905–11911. [Google Scholar] [CrossRef] [PubMed]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Shinoda, Y.; Komura, H.; Homma, S.; Murata, M. Browning of model orange juice solution: Factors affecting the formation of decomposition products. Biosci. Biotechnol. Biochem. 2005, 69, 2129–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurata, T.; Fujimaki, M. Roles of l-xylosone in browning reaction of dehydro-l-ascorbic acid. Agric. Biol. Chem. 1976, 40, 1429–1430. [Google Scholar] [CrossRef]
- Whiting, G.C.; Coggins, R.A. Formation of l-xylosone from ascorbic acid. Nature 1960, 185, 843–844. [Google Scholar] [CrossRef]
- Kurata, T.; Sakurai, Y. Degradation of l-Ascorbic Acid and Mechanism of Nonenzymic Browning Reaction, Part III. Oxidative Degradation of l-Ascorbic Acid (Degradation of Dehydro-l-ascorbic Acid). Agric. Biol. Chem. 1967, 31, 177–184. [Google Scholar] [CrossRef]
- Chumakov, A.; Batalova, V.; Slizhov, Y. Electro-Fenton-like reactions of transition metal ions with electrogenerated hydrogen peroxide. AIP Conf. Proc. 2016, 1772, 040004. [Google Scholar] [CrossRef]
- Rodriguez, M.; Sadler, G.D.; Sims, C.A.; Braddock, R.J. Chemical Changes during Storage of an Alcoholic Orange Juice Beverage. J. Food Sci. 1991, 56, 475–479. [Google Scholar] [CrossRef]
- Finholt, P.; Paulssen, R.B.; Alsos, I.; Higuch, T. Rate Studies on the Anaerobic Degradation of Ascorbic Acid. Ii. Rate of Formation of Carbon Dioxide. J. Pharm. Sci. 1965, 54, 124–128. [Google Scholar] [CrossRef]
- Pastore, P.; Rizzetto, T.; Curcuruto, O.; Cin, M.D.; Zaramella, A.; Marton, D. Characterization of dehydroascorbic acid solutions by liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 2051–2057. [Google Scholar] [CrossRef]
- Sanchita, B.; Salima, V.; Mashenka, Z.; Gerhard, J.H. Inhibition of Streptococcus mutans and Other Oral Streptococci by Hop (Humulus lupulus L.) Constituents. Econ. Bot. 2003, 57, 118–125. [Google Scholar]
- Lschner, J.; Kroh, L.; Vogel, J. l-Ascorbic acid? A carbonyl component of non-enzymatic browning reactions. Z. Fr. Lebensm.-Unters. Und Forsch. 1990, 191, 302–305. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Goshima, K.; Maezono, N.; Tokuyama, K. A Novel Degradation Pathway of L-Ascorbic Acid under Non-oxidative Conditions. Bull. Chem. Soc. Jpn. 1973, 46, 902–904. [Google Scholar] [CrossRef] [Green Version]
- Veli’sek, J.; Davidek, J.; Kubelka, V.; Zelinkova, Z.; Pokorny, J. Volatile degradation products of l-dehydroascorbic acid. Z Lebensm Unters 1976, 162, 285–290. [Google Scholar] [CrossRef]
- Vernin, G.; Chakib, S.; Rogacheva, S.M.; Obretenov, T.D.; Párkányi, C. Thermal decomposition of ascorbic acid. Carbohydr. Res. 1997, 305, 1–15. [Google Scholar] [CrossRef]
- Schulz, A.; Trage, C.; Schwarz, H.; Kroh, L.W. Electrospray ionization mass spectrometric investigations of α-dicarbonyl compounds—Probing intermediates formed in the course of the nonenzymatic browning reaction of l-ascorbic acid. Int. J. Mass Spectrom. 2007, 262, 169–173. [Google Scholar] [CrossRef]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Chen, Q.X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Pourteymour Fard Tabrizi, F.; Kamari, N. Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Rama, R.; Meenakshi, S.; Manjunathan, J.; Abirami, G.; Karthikeyan, S. Furoate Based Functionalised Ionic Liquid: Antimicrobial and Antioxidant Studies. Aust. J. Chem. 2020, 74, 186–191. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Yang, X.; Hu, S. Antioxidant and antibacterial activities of modified crab shell bioactive peptides by Maillard reaction. Int. J. Food Prop. 2018, 21, 2730–2743. [Google Scholar] [CrossRef]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard reaction products as antimicrobial components for packaging films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Q.; Fan, D.; Xiao, J.; Zhao, Y.; Cheng, K.-W.; Wang, M. Novel roles of hydrocolloids in foods: Inhibition of toxic maillard reaction products formation and attenuation of their harmful effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Prince, A.E.; McDonald, D.J.; Roy, S. Exploration of the antimicrobial synergy between selected natural substances on Streptococcus mutans to identify candidates for the control of dental caries. Microbiol. Spectrum 2022, 0, e02357-21. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Ndalane, R.J. The Effects of the Trapping of Methylglyoxal by Flavonoids on Antioxidant and Antibacterial Activity; University of Pretoria: Pretoria, South Africa, 2019. [Google Scholar]
- John, S.; Rafelt, J.H.C. Recent advances in the partial oxidation of organic molecules using heterogeneous catalysis. Catal. Today 2000, 57, 33–44. [Google Scholar]
- Muffler, K.; Leipold, D.; Scheller, M.-C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process. Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Burton, S.G. The search for the ideal biocatalyst. Trends Biotechnol. 2003, 20, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claus, H. Laccases: Structure, reactions, distribution. Micron 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Alcalde, M.; Ballesteros, A. Laccases and their applications: A patent review. Microb. Cell Factories 2008, 7, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flickinger, M.C.; Drew, S.W. Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation; John Wiley & Sons: Minneapolis, MN, USA, 2002. [Google Scholar]
- Kim, S.; Zille, A.; Murkovic, M.; Güebitz, G.; Cavaco-Paulo, A. Enzymatic polymerization on the surface of functionalized cellulose fibers. Enzym. Microb. Technol. 2007, 40, 1782–1787. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzym. Microb. Technol. 2008, 43, 84–92. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Lu, Q.; Zhang, L.; Wang, X.; Liu, R. C-ring cleavage metabolites of catechin and epicatechin enhanced antioxidant activities through intestinal microbiota. Food Res. Int. 2020, 135, 109271–109281. [Google Scholar] [CrossRef]
- Syafni, N. 3,4-dihydroxybenzoic acid and 3,4-dihydroxybenzaldehyde from the fern Trichomanes chinense L.; isolation, antimicrobial and antioxidant properties. Indones. J. Chem. 2012, 12, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Synowiec, A.; Żyła, K.; Gniewosz, M.; Kieliszek, M. An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli. Open Life Sci. 2021, 16, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Thermally Stable and Antimicrobial Active Poly(Catechin) Obtained by Reaction with a Cross-Linking Agent. Biomolecules 2021, 11, 50. [Google Scholar] [CrossRef]
- Sahiner, N. One step poly(quercetin) particle preparation as biocolloid and its characterization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 452, 173–180. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Koorbanally, N.; Kudanga, T. Enzymatic dimerization of luteolin enhances antioxidant and antimicrobial activities. Biocatal. Agric. Biotechnol. 2021, 35, 102105–102115. [Google Scholar] [CrossRef]
- Awuchi, C.G. The Biochemistry, Toxicology, and Uses of the Pharmacologically Active Phytochemicals: Alkaloids, Terpenes, Polyphenols, and Glycosides. J. Food Pharm. Sci. 2019, 7, 1–21. [Google Scholar] [CrossRef]
- Wang, F.; Gong, J.; Zhang, X.; Ren, Y.; Zhang, J. Preparation of biocolorant and eco-dyeing derived from polyphenols based on laccase-catalyzed oxidative polymerization. Polymers 2018, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Noro, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Coloured and low conductive fabrics by in situ laccase-catalysed polymerization. Process. Biochem. 2019, 77, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Liu, X.; Pei, J.; Wang, Y. Grafting of laccase-catalysed oxidation of butyl paraben and p-coumaric acid onto chitosan to improve its antioxidant and antibacterial activities. React. Funct. Polym. 2020, 149, 104511–104517. [Google Scholar] [CrossRef]
- Li, N.; Su, J.; Wang, H.; Cavaco-Paulo, A. Production of antimicrobial powders of guaiacol oligomers by a laccase-catalyzed synthesis reaction. Process. Biochem. 2021, 111, 213–220. [Google Scholar] [CrossRef]
- Ihssen, J.; Schubert, M.; Thony-Meyer, L.; Richter, M. Laccase catalyzed synthesis of iodinated phenolic compounds with antifungal activity. PLoS ONE 2014, 9, e89924–e89936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprio, S.; Sandri, M.; Panseri, S.; Iafisco, M.; Ruffini, A.; Minardi, S.; Tampieri, A. biomineralization. In Bone Substitute Biomaterials; Mallick, K., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 3–29. [Google Scholar]
- Božič, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Koua, D.; Cerutti, L.; Falquet, L.; Sigrist, C.J.; Theiler, G.; Hulo, N.; Dunand, C. PeroxiBase: A database with new tools for peroxidase family classification. Nucleic Acids Res. 2009, 37, D261–D266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth., H.; Saunders, B.C. Studies in peroxidase action. Part, X. The oxidation of phenols. J. Chem. Soc. 1956, 940–948. [Google Scholar] [CrossRef]
- Harris, R.Z.; Newmyer, S.; De Montellano, P.O. Horseradish peroxidase-catalyzed two-electron oxidations. Oxidation of iodide, thioanisoles, and phenols at distinct sites. J. Biol. Chem. 1993, 268, 1637–1645. [Google Scholar] [CrossRef]
- Touch, V.; Hayakawa, S.; Yamada, S.; Kaneko, S. Effects of a lactoperoxidase-thiocyanate-hydrogen peroxide system on Salmonella enteritidis in animal or vegetable foods. Int. J. Food Microbiol. 2004, 93, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Naknukool, S.; Hayakawa, S.; Ogawa, M.; Ni’matulah, A.-B.A. Enhancement of antimicrobial activity of a lactoperoxidase system by carrot extract and β-carotene. Food Chem. 2012, 130, 541–546. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, L.; Duarte-Sierra, A. Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means. Horticulturae 2022, 8, 401. https://doi.org/10.3390/horticulturae8050401
Panda L, Duarte-Sierra A. Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means. Horticulturae. 2022; 8(5):401. https://doi.org/10.3390/horticulturae8050401
Chicago/Turabian StylePanda, Likun, and Arturo Duarte-Sierra. 2022. "Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means" Horticulturae 8, no. 5: 401. https://doi.org/10.3390/horticulturae8050401
APA StylePanda, L., & Duarte-Sierra, A. (2022). Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means. Horticulturae, 8(5), 401. https://doi.org/10.3390/horticulturae8050401