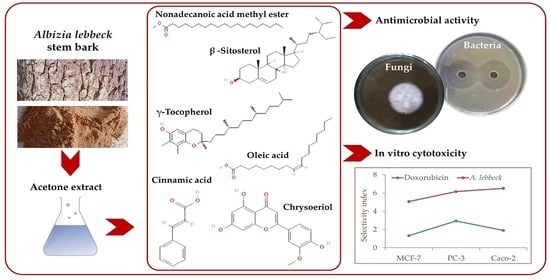
The Acetone Extract of Albizia lebbeck Stem Bark and Its In Vitro Cytotoxic and Antimicrobial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of A. lebbeck Extract
2.3. Extract Derivatization and Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Antibacterial Activity
2.6. Antifungal Activity
2.7. Cytotoxicity Test In Vitro
2.7.1. Cell Cultures
2.7.2. MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide) Assay
2.8. Statistical Analysis
3. Results
3.1. GC-MS Analysis
3.2. HPLC Analysis
3.3. Antibacterial Activity
3.4. Antifungal Activity
3.5. In Vitro Cytotoxic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.S.; Gothecha, V.K.; Sharma, A. Albizia lebbeck: A short review. J. Herb. Med. Toxicol. 2010, 4, 9–15. [Google Scholar]
- Amog, P.U.; Manjuprasanna, V.N.; Yariswamy, M.; Nanjaraj Urs, A.N.; Joshi, V.; Suvilesh, K.N.; Nataraju, A.; Vishwanath, B.S.; Gowda, T.V. Albizia lebbeck seed methanolic extract as a complementary therapy to manage local toxicity of Echis carinatus venom in a murine model. Pharm. Biol. 2016, 54, 2568–2574. [Google Scholar] [CrossRef] [Green Version]
- Rashid, R.B.; Chowdhury, R.; Jabbar, A.; Hasan, C.M.; Rashid, M.A. Constituents of Albizzia lebbeck and antibacterial activity of an isolated flavone derivative. Saudi Pharm. J 2003, 11, 52–56. [Google Scholar]
- Noté, O.P.; Jihu, D.; Antheaume, C.; Zeniou, M.; Pegnyemb, D.E.; Guillaume, D.; Chneiwess, H.; Kilhoffer, M.C.; Lobstein, A. Triterpenoid saponins from Albizia lebbeck (L.) Benth and their inhibitory effect on the survival of high grade human brain tumor cells. Carbohydr. Res. 2015, 404, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, M.; Kundu, S.S.; Singh, S.; Misra, A.K. Cornell net carbohydrate and protein system for nutritional evluation of tree leaves, shrubs and grasses. Indian J Anim Sci. 2006, 76, 81–87. [Google Scholar]
- Varshney, I.P. Glycosides and carbohydrates from the members of the family Leguminosae. Univ. Indore Res. J. Sci. 1976, 4, 13–22. [Google Scholar]
- Pal, B.C.; Achari, B.; Yoshikawa, K.; Arihara, S. Saponins from Albizia lebbeck. Phytochemistry 1995, 38, 1287–1291. [Google Scholar] [CrossRef]
- Wati, M.; Khabiruddin, M. Comparision of antioxidants in phenol extract and methanol extract of Albizia lebbeck from two locations. Int. J. Pharm. Sci. Rev. Res. Int. 2017, 45, 78–82. [Google Scholar]
- Elshiekh, Y.H.; Alagbash, R.E.; Ali, R.A.; Saad, F.O.; Musharaf, M. Phytochemical constituents, antibacterial screening and antioxidant activity of Albizia lebbeck (L.) Benth (Seed). N. A. J. Adv. Res. Rev. 2020, 7, 035–040. [Google Scholar]
- Babu, N.P.; Pandikumar, P.; Ignacimuthu, S. Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol. 2009, 125, 356–360. [Google Scholar] [CrossRef]
- Gul, F.; Shinwari, Z.K.; Afzal, I. Screening of indigenous knowledge of herbal remedies for skin diseases among local communities of north West Punjab, Pakistan. Pak. J. Bot. 2012, 44, 1609–1616. [Google Scholar]
- Farag, M.; El Gamal, A.; Kalil, A.; Al-Rehaily, A.; El Mirghany, O.; El Tahir, K. Evaluation of some biological activities of Albizia lebbeck flowers. Pharmacol. Pharm. 2013, 4, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Ghany, A.E.-S.; Dora, G.; Abdallah, R.H.; Hassan, W.; El-Salam, E.A. Phytochemical and biological study of Albizia lebbeck stem bark. J Chem Pharma Res 2015, 7, 29–43. [Google Scholar]
- Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Vincent Bensy, A.D.; Rajaselvam, J. Bioactive potential of Albizia lebbeck extract against phytopathogens and protective properties on tomato plant against speck disease in greenhouse. Physiol. Mol. Plant Pathol. 2022, 117, 101750. [Google Scholar] [CrossRef]
- Suruse, P.B.; Bodele, S.B.; Duragkar, N.J.; Saundankar, Y.G. In-Vitro evaluation of antioxidant activity of Albizia Lebbeck bark. Int. J. Biol. Sci. Ayuveda Res. 2013, 1, 6–17. [Google Scholar]
- Abdul-Hafeez, E.Y.; Nguyen, T.N.; Karamova, N.S.; Ilinskaya, O.N. Antibacterial activity of certain medicinal plants on different bacterial strains associated with colorectral cancer. Int. J. Biosci. 2014, 5, 219–229. [Google Scholar]
- Ali, M.T.; Haque, S.T.; Kabir, M.L.; Rana, S.; Haque, M.E. A comparative study of in vitro antimicrobial, antioxidant and cytotoxic activity of Albizia lebbeck and Acacia nilotica stem bark. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 34–38. [Google Scholar] [CrossRef]
- El-Hawary, S.; El-Fouly, K.; Sokkar, N.M.; Talaat, Z. A phytochemical profile of Albizia lebbeck (L.) Benth. cultivated in Egypt. Asian J. Biochem. 2011, 6, 122–141. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Hafeez, E.Y.; Karamova, N.S.; Ilinskaya, O.N. Antioxidant activity and total phenolic compound content of certain medicinal plants. Int. J. Biosci. 2014, 5, 213–222. [Google Scholar]
- Desai, S.; Tatke, P.; Gabhe, S. Isolation of catechin from stem bark of Albizia lebbeck. Int. J. Res. Pharm. Biomed. Sci. 2014, 3, 31–35. [Google Scholar]
- Ahmed, D.; Kumar, V.; Sharma, M.; Verma, A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Complement Altern. Med. 2014, 14, 155. [Google Scholar] [CrossRef] [Green Version]
- Desai, T.H.; Joshi, S.V. Anticancer activity of saponin isolated from Albizia lebbeck using various in vitro models. J. Ethnopharmacol. 2019, 231, 494–502. [Google Scholar] [CrossRef]
- Viana, E.O.R.; Cruz, M.d.F.S.J.; da Silva, M.J.; Pereira, G.M.; da Silva, B.P.; Tinoco, L.W.; Parente, J.P. Structural characterization of a complex triterpenoid saponin from Albizia lebbeck and investigation of its permeability property and supramolecular interactions with membrane constituents. Carbohydr. Res. 2019, 471, 105–114. [Google Scholar] [CrossRef]
- Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Mahmoud, A.F. Antimicrobial and Antioxidant Activities of Stem Bark Extracts of Different Ornamental Trees. Assiut J. Agric. Sci 2015, 46, 19–32. [Google Scholar]
- Thakur, R.S.; Ahirwar, B. A steroidal derivative from Trigonella foenum graecum L. that induces apoptosis in vitro and in vivo. J Food Drug Anal. 2019, 27, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Noté, O.P.; Ngo Mbing, J.; Kilhoffer, M.-C.; Pegnyemb, D.E.; Lobstein, A. Lebbeckoside C, a new triterpenoid saponin from the stem barks of Albizia lebbeck inhibits the growth of human glioblastoma cells. Nat. Prod. Res. 2019, 33, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Grattan, B.J. Plant Sterols as Anticancer Nutrients: Evidence for Their Role in Breast Cancer. Nutrients 2013, 5, 359–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Kumar, P.; Jindal, A. Antibacterial potential of sterols of some medicinal plants. Int. J. Pharm. Pharm. Sci. 2012, 4, 159–162. [Google Scholar]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytoscience 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Ali, A.; Qadir, A.; Ali, A.; Warsi, M.H.; Tahir, A.; Ali, A. GC-MS analysis and antioxidant activity of Wrightia tinctoria R.Br. leaf extract. J. AOAC Int. 2021, 104, 1415–1419. [Google Scholar] [CrossRef]
- Jangwan, J.S.; Dobhal, M.; Kumar, N. New cytotoxic saponin of Albizzia lebbeck. Indian J. Chem. B 2010, 49, 123–126. [Google Scholar]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharm. 2020, 871, 172937. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, S.; Menzel, A. A subchronic 90-day oral rat toxicity study and in vitro genotoxicity studies with a conjugated linoleic acid product. Food Chem. Toxicol. 2003, 41, 1749–1760. [Google Scholar] [CrossRef]
- Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M.S.; Saravanan, M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021, 1235, 130229. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Arora, S.; Kumar, G. Phytochemical screening of root, stem and leaves of Cenchrus biflorus Roxb. J. Pharm. Phytochem. 2018, 7, 1445–1450. [Google Scholar]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of tongue squamous cell carcinomas. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yff, B.T.S.; Lindsey, K.L.; Taylor, M.B.; Erasmus, D.G.; Jäger, A.K. The pharmacological screening of Pentanisia prunelloides and the isolation of the antibacterial compound palmitic acid. J. Ethnopharmacol. 2002, 79, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Ngamakeue, N.; Chitprasert, P. Encapsulation of Holy Basil Essential Oil in Gelatin: Effects of Palmitic Acid in Carboxymethyl Cellulose Emulsion Coating on Antioxidant and Antimicrobial Activities. Food Bioproc. Tech. 2016, 9, 1735–1745. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Novak, A.F.; Clark, G.C.; Dupuy, H.P. Antimicrobial activity of some ricinoleic acid oleic acid derivatives. JAOCS 1961, 38, 321–324. [Google Scholar] [CrossRef]
- Brulez, W.; Zeller, W. Seasonal changes of epiphytic Erwinia amylovora on ornamentals in relation to weather conditions and course of infections. Acta Hortic. 1981, 117, 37–43. [Google Scholar]
- Ibrahim, O.H.M.; Abo-Elyousr, K.A.M.; Asiry, K.A.; Alhakamy, N.A.; Mousa, M.A.A. Phytochemical characterization, antimicrobial activity and in vitro antiproliferative potential of Alchemilla vulgaris Auct root extract against prostate (PC-3), breast (MCF-7) and colorectal adenocarcinoma (Caco-2) cancer cell lines. Plants 2022, 11, 2140. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.I.I.; Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R. Effectiveness of plant extracts to control purple blotch and Stemphylium blight diseases of onion (Allium cepa L.) in Assiut, Egypt. Arch. Phytopathol. Plant Prot. 2014, 47, 377–387. [Google Scholar] [CrossRef]
- Ibrahim, O.H.M.; Mousa, M.A.A.; Asiry, K.A.; Alhakamy, N.A.; Abo-Elyousr, K.A.M. Azadirachta indica A. Juss fruit mesocarp epicarp extracts induce, antimicrobial antiproliferative effects against prostate, breast colorectal adenocarcinoma cancer cell lines through upregulation of proapoptotic genes. Plants 2022, 11, 1990. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sakshi; Chauhan, M.; Dabas, A.; Arya, V. A Comprehensive Insight into the Phytochemical, Pharmacological Potential, and Traditional Medicinal Uses of Albizia lebbeck (L.) Benth. Evid. Based Complement. Altern. Med. 2022, 2022, 5359669. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Galal, B.; Nafie, M.S.; El Bous, M.M.; El-Bana, M.I. Cytotoxic, apoptotic activities and chemical profiling of dimorphic forms of Egyptian halophyte Cakile maritima scop. J. Biomol. Struct. Dyn. 2023, 41, 147–160. [Google Scholar] [CrossRef] [PubMed]






| No. | Compound | Retention Time (min) | Peak Area % | Chemical Formula | Molecular Weight | CAS Number |
|---|---|---|---|---|---|---|
| 1 | 1,4-Benzenediol, 2-(1,1-dimethylethyl)-5-(2-propenyl)- “2-Allyl-5-t-butylhydroquinone” | 17.64 | 3.33 | C13H18O2 | 206 | 73685-60-6 |
| 2 | Methyl-9,9,10,10-D4-octadecanoate | 22.25 | 0.84 | C19H34D4O2 | 302 | 56554-85-9 |
| 3 | Isopropyl myristate “Tetradecanoic acid, 1-methylethyl ester” | 24.33 | 1.17 | C17H34O2 | 270 | 110-27-0 |
| 4 | 1,25-Dihydroxyvitamin D3, TMS derivative | 24.86 | 0.65 | C30H52O3Si | 488 | 55759-94-9 |
| 5 | Isochiapin B | 26.12 | 0.65 | C19H22O6 | 346 | NA |
| 6 | Hexadecanoic acid, methyl ester “Palmitic acid, methyl ester” | 26.29 | 1.77 | C17H34O2 | 270 | 112-39-0 |
| 7 | Hexadecanoic acid “Palmitic Acid” | 27.15 | 3.73 | C16H32O2 | 256 | 57-10-3 |
| 8 | Hexadecanoic acid, trimethylsilyl ester “Trimethylsilyl palmitate” | 28.6 | 1.69 | C19H40O2Si | 328 | 55520-89-3 |
| 9 | 7,10-Octadecadienoic acid, methyl ester | 29.37 | 2.31 | C19H34O2 | 294 | 56554-24-6 |
| 10 | 9-Octadecenoic acid (z)-, methyl ester “Oleic acid, methyl ester” | 29.49 | 6.69 | C19H36O2 | 296 | 1937-62-8 |
| 11 | 9-Octadecenoic acid (z)- “Oleic acid” | 29.61 | 1.17 | C18H34O2 | 282 | 112-80-1 |
| 12 | Methyl-9,9,10,10-D4-octadecanoate | 29.98 | 1.25 | C19H34D4O2 | 302 | 56554-85-9 |
| 13 | 9-Octadecenoic acid (z)- “Oleic acid” | 30.28 | 8.92 | C18H34O2 | 282 | 112-80-1 |
| 14 | 9-Octadecenoic acid (z)- “Oleic acid” | 30.71 | 2.42 | C18H34O2 | 282 | 112-80-1 |
| 15 | 9,12-Octadecadienoic acid (z,z)-, methyl ester “Methyl linoleate” | 31.05 | 0.77 | C19H34O2 | 294 | 112-63-0 |
| 16 | 2-Hydroxy-3-[(9E)-9-octadecenoyloxy]propyl | 34.95 | 1.64 | C39H72O5 | 620 | 2465-32-9 |
| 17 | 1-Heptatriacotanol | 35.59 | 0.8 | C37H76O | 536 | 105794-58-9 |
| 18 | 2-Hydroxy-3-[(9E)-9-octadecenoyloxy] propyl (9E)-9-octadecenoate | 35.66 | 1.38 | C39H72O5 | 620 | 2465-32-9 |
| 19 | Isochiapin B | 36.77 | 1.43 | C19H22O6 | 346 | NA |
| 20 | .psi.,.psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-dimethoxy- “3,4,3′,4′-Tetrahydrospirilloxanthin” | 37.09 | 1.72 | C42H64O2 | 600 | 13833-01-7 |
| 21 | γ-Tocopherol | 37.64 | 8.65 | C28H48O2 | 416 | 7616-22-0 |
| 22 | Ethyl iso-allocholate | 37.9 | 4.58 | C26H44O5 | 436 | NA |
| 23 | 9,12,15-Octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester, (z,z,z)- | 38.33 | 1.19 | C27H52O4Si2 | 496 | 55521-22-7 |
| 24 | 2-Hydroxy-3-[(9E)-9-octadecenoyloxy] propyl (9E)-9-octadecenoate | 39.58 | 3.16 | C39H72O5 | 620 | 2465-32-9 |
| 25 | Stigmast-5-en-3-ol, (3beta)- “β -Sitosterol” | 40.46 | 11.84 | C29H50O | 414 | 83-47-5 |
| 26 | 2,6-Bis(3,4-methylenedioxyphenyl)-3,7-dioxabicyclo(3.3.0)octane | 40.71 | 3.62 | C20H18O6 | 354 | 7076-24-6 |
| 27 | Dodecanoic acid methyl ester “Methyl laurate” | 40.88 | 6.3 | C13H26O2 | 214 | 111-82-0 |
| 28 | Nonadecanoic acid methyl ester | 41.04 | 11.66 | C20H40O2 | 313 | 1731-94-8 |
| 29 | Docosanoic acid methyl ester “Methyl behenate” | 41.33 | 2.37 | C23H77O2 | 355 | 929-77-1 |
| 30 | Gitoxigenin | 41.50 | 0.83 | C23H34O5 | 390 | 545-26-6 |
| 31 | 4-Methoxy-hexacosanoic acid | 41.54 | 1.48 | C26H52O2 | 397 | 506-46-7 |
| No. | Compound | Retention Time Min | Concentration | ||
|---|---|---|---|---|---|
| μg/mL Acetone Extract | µg/g Dry Extract | μg/g Stem Bark DW | |||
| 1 | Catechol | 4.5 | 7.22 | 144.4 | 6.9 |
| 2 | Syringic acid | 5.1 | 5.36 | 107.2 | 5.1 |
| 3 | Cinnamic acid | 7.0 | 29.63 | 592.6 | 28.3 |
| 4 | Caffeic acid | 8.0 | 5.47 | 109.4 | 5.2 |
| 5 | Gallic acid | 9.8 | 5.06 | 101.2 | 4.8 |
| 6 | Salicylic acid | 12.0 | 6.33 | 126.6 | 6.1 |
| 7 | Ellagic acid | 12.8 | 14.96 | 299.2 | 14.3 |
| 8 | Protocatechuic acid | 15.6 | 2.36 | 47.2 | 2.3 |
| No. | Compound | Retention Time Min | Concentration | ||
|---|---|---|---|---|---|
| μg/mL Acetone Extract | µg/g Dry Extract | μg/g Stem Bark DW | |||
| 1 | Naringin | 4.6 | 7.14 | 142.8 | 6.8 |
| 2 | Rutin | 5.2 | 6.16 | 123.2 | 5.9 |
| 3 | Quercetin | 6.9 | 11.41 | 228.2 | 10.9 |
| 4 | Kaempferol | 8.1 | 4.17 | 83.4 | 4.0 |
| 5 | Luteolin | 9.0 | 6.13 | 122.6 | 5.9 |
| 6 | Hisperdin | 10.0 | 14.45 | 289 | 13.8 |
| 7 | Catechin | 12.01 | 7.14 | 142.8 | 6.8 |
| 8 | Chrysoeriol | 15.0 | 24.08 | 481.6 | 23.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, O.H.M.; Abdul-Hafeez, E.Y. The Acetone Extract of Albizia lebbeck Stem Bark and Its In Vitro Cytotoxic and Antimicrobial Activities. Horticulturae 2023, 9, 385. https://doi.org/10.3390/horticulturae9030385
Ibrahim OHM, Abdul-Hafeez EY. The Acetone Extract of Albizia lebbeck Stem Bark and Its In Vitro Cytotoxic and Antimicrobial Activities. Horticulturae. 2023; 9(3):385. https://doi.org/10.3390/horticulturae9030385
Chicago/Turabian StyleIbrahim, Omer H. M., and Essam Y. Abdul-Hafeez. 2023. "The Acetone Extract of Albizia lebbeck Stem Bark and Its In Vitro Cytotoxic and Antimicrobial Activities" Horticulturae 9, no. 3: 385. https://doi.org/10.3390/horticulturae9030385
APA StyleIbrahim, O. H. M., & Abdul-Hafeez, E. Y. (2023). The Acetone Extract of Albizia lebbeck Stem Bark and Its In Vitro Cytotoxic and Antimicrobial Activities. Horticulturae, 9(3), 385. https://doi.org/10.3390/horticulturae9030385