Mn‐Containing Paramagnetic Conductors with Bis(ethylenedithio)tetrathiafulvalene (BEDT‐TTF)
Abstract
:1. Introduction
- (i)
- Monomers as in (TTF)7[Fe(C2O4)3]2·4H2O [17], (TTF)3[Ru(C2O4)3]·(EtOH)0.5·4H2O [18], (BEST)4[M(C2O4)3]·PhCOOH·H2O [19], (BEST)4[M(C2O4)3]·1.5H2O [19], (M = Cr and Fe), (BEST)9[Fe(C2O4)3]2·7H2O [19], (ET)2[Ge(C2O4)3]·PhCN [20], (ET)9Na18[M(C2O4)3]8·24H2O (MIII = Fe and Cr) [21,22], (ET)12[Fe(C2O4)3]2·nH2O [23], (ET)5[Fe(C2O4)3]·CH2Cl2·2H2O [24], (ET)5[Ge(C2O4)3]2 [25] and (ET)7[Ge(C2O4)3](CH2Cl2)0.87(H2O)0.09 [25]. (TTF = tetrathiafulvalene; BEST = bis(ethylenediseleno)-tetrathiafulvalene; ET = bis(ethylenedithio)tetrathiafulvalene).
- (ii)
- (iii)
- (iv)
- Forming honeycomb-like 2D anionic layers as in the first molecular ferromagnetic metals: (ET)3[MnIICrIII(C2O4)3] and (BETS)x[MnIICrIII(C2O4)3]·CH2Cl2 (BETS = bis(ethylenedithio)tetraselenafulvalene; x ≈ 3) [4,16,29,30,31], and also in the Day’s series of paramagnetic superconductors, metals and semiconductors formulated as (ET)4[AIMIII(C2O4)3]·G (AI = H3O+, K+ and NH4+; MIII = Cr, Fe, Ga, Co, Mn and Al; G = PhCN, PhNO2, py, PhCl2, PhF, PhCl, PhBr, PhCOCH3, PhCH2OHCH3, Me2NCHO, CH2Cl2, PhN(CH3)CHO, PhCH2CN, …) [1]. This series constitutes, by far, the largest family of paramagnetic superconductors, metals and semiconductors prepared to date. In this series, we can distinguish three different crystal structures: (i) a C2/c (#15) monoclinic β′′ phase (Table 1); (ii) an orthorhombic Pbcn (#60) pseudo-κ phase (Table 2) and (iii) a triclinic P1 (#1) or P-1 (#2) αβ′′ or α-pseudo-κ phase (Table 3). Besides these three 4:1 series, there is a fourth series with 3:1 cation:anion stoichiometry with either triclinic P1 (#1), monoclinic P21 (#4) and P21/c (#14) or orthorhombic P212121 (#19) crystal structures (Table 4). The main difference between these four series lies in the disposition of the organic molecules in the cationic layers. The monoclinic C2/c (#15) β′′ phase presents parallel ET molecules, the orthorhombic Pbcn (#60) pseudo-κ phase contains ET dimers surrounded by six monomers, the triclinic phase presents a mixture of alternating θ and β′′ (or θ and pseudo-κ) layers, and, finally, the 3:1 salts present alternating tilted dimers and monomers. These structural differences lead to different physical properties: the triclinic and orthorhombic phases are semiconductors (Table 2, Table 3 and Table 4), whereas the monoclinic salts are metallic or even superconductors (Table 1).
2. Results and Discussion
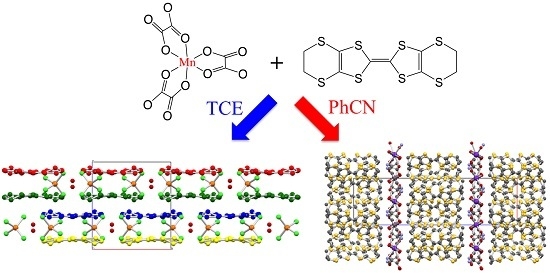
2.1. Syntheses of the Complexes
2.2. Description of the Structures
2.3. Magnetic Properties
2.4. Electrical Properties
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of (ET)4[KMn(C2O4)3]·PhCN (1)
3.3. Synthesis of (ET)[MnCl4]·H2O (2)
3.4. Physical Measurements
3.5. Crystallographic Data Collection and Refinement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Miyazaki, A. Magnetic TTF-Based Charge-Transfer Complexes. Chem. Rev. 2004, 104, 5449–5478. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Cui, H.; Kobayashi, A. Organic Metals and Superconductors Based on BETS (BETS = Bis(ethylenedithio)tetraselenafulvalene). Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kobayashi, A.; Cassoux, P. BETS as a source of molecular magnetic superconductors (BETS = bis(ethylenedithio)tetraselenafulvalene). Chem. Soc. Rev. 2000, 29, 325–333. [Google Scholar] [CrossRef]
- Graham, A.W.; Kurmoo, M.; Day, P. β′′-(bedt-ttf)4[(H2O)Fe(C2O4)3]·PhCN: The first molecular superconductor containing paramagnetic metal ions. J. Chem. Soc. Chem. Commun. 1995, 2061–2062. [Google Scholar] [CrossRef]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3·C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fujiwara, E.; Fujiwara, H.; Tanaka, H.; Tamura, I.; Bin, Z.; Gritsenko, V.; Otsuka, T.; Kobayashi, A.; Tokumoto, M.; et al. Magnetic organic superconductors based on BETS molecules—Interplay of conductivity and magnetism. Mol. Cryst. Liq. Cryst. 2002, 379, 9–18. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tomita, H.; Naito, T.; Kobayashi, A.; Sakai, F.; Watanabe, T.; Cassoux, P. New BETS conductors with magnetic anions (BETS = bis(ethylenedithio)-tetraselenafulvalene). J. Am. Chem. Soc. 1996, 118, 368–377. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fujiwara, E.; Fujiwara, H.; Tanaka, H.; Otsuka, T.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetic organic superconductors, BETS2FeX4 (X = Br, Cl). Mol. Cryst. Liq. Cryst. 2002, 380, 139–144. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fujiwara, E.; Nakazawa, Y.; Narymbetov, B.Z.; Kato, K.; Kobayashi, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. A novel antiferromagnetic organic superconductor κ-(BETS)2FeBr4 [where BETS = bis(ethylenedithio)tetraselenafulvalene]. J. Am. Chem. Soc. 2001, 123, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, H.; Ojima, E.; Fujiwara, H.; Nakazawa, Y.; Otsuka, T.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetism and superconductivity of BETS conductors with Fe3+ ions. Synth. Met. 2001, 120, 663–666. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanaka, H.; Ojima, E.; Fujiwara, H.; Otsuka, T.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Coexistence of antiferromagnetic order and superconductivity in organic conductors. Polyhedron 2001, 20, 1587–1592. [Google Scholar] [CrossRef]
- Tanaka, H.; Kobayashi, H.; Kobayashi, A.; Cassoux, P. Superconductivity, antiferromagnetism, and phase diagram of a series of organic conductors: λ-(BETS)2FexGa1−xBryCl4−y. Adv. Mater. 2000, 12, 1685–1689. [Google Scholar] [CrossRef]
- Ojima, E.; Fujiwara, H.; Kato, K.; Kobayashi, H.; Tanaka, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetic organic metal exhibiting superconducting transition, κ-(BETS)2FeBr4 [BETS = bis(ethylenedithio)tetraselenafulvalene]. J. Am. Chem. Soc. 1999, 121, 5581–5582. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J. A molecular metal ferromagnet from the organic donor bis(ethylenedithio)-tetraselenafulvalene and bimetallic oxalate complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J. Charge transfer salts of tetrathiafulvalene derivatives with magnetic iron(III) oxalate complexes: [TTF]7[Fe(ox)3]2·4H2O, [TTF]5[Fe2(ox)5]·2PhMe·2H2O and [TMTTF]4[Fe2(ox)5]·PhCN·4H2O (TMTTF = tetramethyltetrathiafulvalene). J. Chem. Soc. Dalton Trans. 2000, 205–210. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Martínez-Agudo, J.M.; Martínez-Ferrero, E. Magnetic properties of hybrid molecular materials based on oxalato complexes. Polyhedron 2003, 22, 2381–2386. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J.; Alberola, A. Radical salts of bis(ethylenediseleno)tetrathiafulvalene with paramagnetic tris(oxalato)metalate anions. Inorg. Chem. 2006, 45, 10815–10824. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Uruichi, M.; Yakushi, K. Synthesis, crystal structure and properties of the semiconducting molecular charge-transfer salt (bedt-ttf)2Ge(C2O4)3·PhCN [bedt-ttf = bis(ethylenedithio)tetrathiafulvalene]. J. Mater. Chem. 1999, 9, 2731–2736. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Bingham, A.; Hursthouse, M.B.; McMillan, P.; Firth, S. Multi-layered molecular charge-transfer salts containing alkali metal ions. J. Mater. Chem. 2007, 17, 3324–3329. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P. A molecular charge transfer salt of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallised from a chiral solvent. CrystEngComm 2010, 12, 1369–1372. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Barnett, S.A.; Tocher, D.A.; Horton, P.N.; Hursthouse, M.B. Magnetic molecular charge-transfer salts containing layers of water and tris(oxalato)ferrate(III) anions. CrystEngComm 2008, 10, 192–196. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, F.; Guo, Y. Synthesis, crystal structure, and characterization of charge-transfer salt: (BEDT-TTF)5[Fe(C2O4)3]·(H2O)2·CH2Cl2 (BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). CrystEngComm 2009, 11, 2523–2528. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P.N. BEDT-TTF Tris(oxalato)germanate(IV) Salts with Novel Donor Packing Motifs. Bull. Chem. Soc. Jpn. 2010, 83, 419–423. [Google Scholar] [CrossRef]
- Rashid, S.; Turner, S.S.; Day, P.; Light, M.E.; Hursthouse, M.B. Molecular charge-transfer salt of BEDT-TTF [bis(ethylenedithio)tetrathiafulvalene] with the oxalate-bridged dimeric anion [Fe2(C2O4)5]4−. Inorg. Chem. 2000, 39, 2426–2428. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Ruiz-Perez, C. Hybrid organic/inorganic molecular materials formed by tetrathiafulvalene radicals and magnetic trimeric clusters of dimetallic oxalate-bridged complexes: The series (TTF)4{MII(H2O)2[MIII(ox)3]2}·nH2O (MII = Mn, Fe, Co, Ni, Cu and Zn; MIII = Cr and Fe; ox = C2O42−). Eur. J. Inorg. Chem. 2003, 2290–2298. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Ruiz-Pérez, C.; Triki, S. Hybrid molecular materials formed by alternating layers of bimetallic oxalate complexes and tetrathiafulvalene molecules: Synthesis, structure, and magnetic properties of TTF4{Mn(H2O)2[Cr(ox)3]2}·14H2O. Adv. Mater. 1996, 8, 737–740. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Romero, F.M. Multifunctionality in hybrid molecular materials: Design of ferromagnetic molecular metals and hybrid magnets. Synth. Met. 2003, 133, 509–513. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R.; Coronado, E.; Goddard, P.A.; Singleton, J.; Coldea, A.I.; Wallis, J.D.; Coles, S.J.; Alberola, A. A Chiral Ferromagnetic Molecular Metal. J. Am. Chem. Soc. 2010, 132, 9271–9273. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R. Hybrid molecular conductors. J. Mater. Chem. 2005, 15, 66–74. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Hibbs, D.E.; Light, M.E.; Hursthouse, M.B.; Uruichi, M.; Yakushi, K. Crystal Chemistry and Physical Properties of Superconducting and Semiconducting Charge Transfer Salts of the Type (BEDT-TTF)4[AIMIII(C2O4)3]·PhCN (AI = H3O, NH4, K; MIII = Cr, Fe, Co, Al; BEDT-TTF = Bis(ethylenedithio)tetrathiafulvalene). Inorg. Chem. 2001, 40, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Akutsu-Sato, A.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Day, P. Suppression of superconductivity in a molecular charge transfer salt by changing guest molecule: β′′-(BEDT-TTF)4[(H3O)Fe(C2O4)3](C6H5CN)x(C5H5N)1−x. J. Mater. Chem. 2007, 17, 2497–2499. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Korobenko, A.V.; Zverev, V.N. Effect of electrocrystallization medium on quality, structural features, and conducting properties of single crystals of the (BEDT-TTF)4AI[FeIII(C2O4)3]·G family. CrystEngComm 2011, 13, 537–545. [Google Scholar] [CrossRef]
- Turner, S.S.; Day, P.; Malik, K.M.A.; Hursthouse, M.B.; Teat, S.J.; MacLean, E.J.; Martin, L.; French, S.A. Effect of included solvent molecules on the physical properties of the paramagnetic charge transfer salts β′′-(bedt-ttf)4[(H3O)Fe(C2O4)3]·solvent (bedt-ttf = bis(ethylenedithio)tetrathiafulvalene). Inorg. Chem. 1999, 38, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Turner, S.S.; Day, P.; Howard, J.A.K.; Guionneau, P.; McInnes, E.J.L.; Mabbs, F.E.; Clark, R.J.H.; Firth, S.; Biggs, T. New superconducting charge-transfer salts (BEDT-TTF)4[A·M(C2O4)3]·C6H5NO2 (A = H3O or NH4, M = Cr or Fe, BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). J. Mater. Chem. 2001, 11, 2095–2101. [Google Scholar] [CrossRef]
- Sun, S.Q.; Wu, P.J.; Zhang, Q.C.; Zhu, D.B. The New Semiconducting Magnetic Charge Transfer Salt (BEDT-TTF)4·H2O·Fe(C2O4)3·C6H5NO2: Crystal Structure and Physical Properties. Mol. Cryst. Liq. Cryst. 1998, 319, 259–269. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P.; Zhang, Q.; Zhu, D. The new semiconducting magnetic charge transfer salt (BEDT-TTF)4·H2O·Fe(C2O4)3·C6H5NO2: Crystal structure and physical properties. Synth. Met. 1998, 94, 161–166. [Google Scholar] [CrossRef]
- Zorina, L.; Prokhorova, T.; Simonov, S.; Khasanov, S.; Shibaeva, R.; Manakov, A.; Zverev, V.; Buravov, L.; Yagubskii, E. Structure and magnetotransport properties of the new quasi-two-dimensional molecular metal β′′-(BEDT-TTF)4H3O[Fe(C2O4)3]·C6H4Cl2. J. Exp. Theor. Phys. 2008, 106, 347–354. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. A novel paramagnetic molecular superconductor formed by bis(ethylenedithio)tetrathiafulvalene, tris(oxalato) ferrate(III) anions and bromobenzene as guest molecule: ET4[(H3O)Fe(C2O4)3]·C6H5Br. J. Mater. Chem. 2005, 15, 1429–1436. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Khasanov, S.S.; Zorina, L.V.; Buravov, L.I.; Tkacheva, V.A.; Baskakov, A.A.; Morgunov, R.B.; Gener, M.; Canadell, E.; Shibaeva, R.P.; et al. Molecular Metals Based on BEDT-TTF Radical Cation Salts with Magnetic Metal Oxalates as Counterions: β′′-(BEDT-TTF)4A[M(C2O4)3]·DMF (A = NH4+, K+; M = CrIII, FeIII). Adv. Funct. Mater. 2003, 13, 403–411. [Google Scholar] [CrossRef]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Bulanchuk, P.O.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Structural phase transition in the β′′-(BEDT-TTF)4H3O[Fe(C2O4)3]·G crystals (where G is a guest solvent molecule). CrystEngComm 2012, 14, 460–465. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. The series of molecular conductors and superconductors ET4[AFe(C2O4)3]·PhX (ET = bis(ethylenedithio)tetrathiafulvalene; (C2O4)2− = oxalate; A+ = H3O+, K+; X = F, Cl, Br, and I): Influence of the halobenzene guest molecules on the crystal structure and superconducting properties. Inorg. Chem. 2012, 51, 1111–1126. [Google Scholar] [PubMed]
- Akutsu-Sato, A.; Kobayashi, A.; Mori, T.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Day, P.; Tocher, D.A.; Light, M.E.; et al. Structures and Physical Properties of New β′-BEDT-TTF Tris-Oxalatometallate (III) Salts Containing Chlorobenzene and Halomethane Guest Molecules. Synth. Met. 2005, 152, 373–376. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Gimenez-Saiz, C.; Gómez-García, C.J. New magnetic conductors and superconductors based on BEDT-TTF and BEDS-TTF. Synth. Met. 2005, 154, 245–248. [Google Scholar] [CrossRef]
- Kanehama, R.; Yoshino, Y.; Ishii, T.; Manabe, T.; Hara, H.; Miyasaka, H.; Matsuzaka, H.; Yamashita, M.; Katada, M.; Nishikawa, H.; et al. Syntheses and physical properties of new charge-transfer salts consisting of a conducting BEDT-TTF column and magnetic 1D or 2D Fe(III) networks. Synth. Met. 2003, 133, 553–554. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Malik, K.M.A.; Coles, S.J.; Hursthouse, M.B. Polymorphism based on molecular stereoisomerism in tris(oxalato) Cr(III) salts of bedt-ttf [bis(ethylenedithio)tetrathiafulvalene]. Chem. Commun. 1999, 513–514. [Google Scholar] [CrossRef]
- Rashid, S.; Turner, S.S.; Le Pevelen, D.; Day, P.; Light, M.E.; Hursthouse, M.B.; Firth, S.; Clark, R.J.H. β′′-(BEDT-TTF)4[(H3O)Cr(C2O4)3]CH2Cl2: Effect of included solvent on the structure and properties of a conducting molecular charge-transfer salt. Inorg. Chem. 2001, 40, 5304–5306. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Le Pevelen, D.; Day, P.; Laukhin, V.; Klehe, A.; Singleton, J.; Tocher, D.A.; Probert, M.R.; et al. Effect of included guest molecules on the normal state conductivity and superconductivity of β′′-(ET)4[(H3O)Ga(C2O4)3]·G (G = pyridine, nitrobenzene). J. Am. Chem. Soc. 2002, 124, 12430–12431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N. Metallic Bi- and Monolayered Radical Cation Salts Based on Bis(ethylenedithio)-tetrathiafulvalene (BEDT-TTF) with the Tris(oxalato)gallate Anion. Eur. J. Inorg. Chem. 2014, 3933–3940. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Canadell, E.; Shibaeva, R.P.; Yagubskii, E.B. The first molecular superconductor based on BEDT-TTF radical cation salt with paramagnetic tris(oxalato)ruthenate anion. CrystEngComm 2013, 15, 7048–7055. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Canadell, E. Effect of Halopyridine Guest Molecules on the Structure and Superconducting Properties of β′′-[Bis(ethylenedithio)tetrathiafulvalene]4(H3O)[Fe(C2O4)3]·Guest Crystals. Eur. J. Inorg. Chem. 2015, 2015, 5611–5620. [Google Scholar] [CrossRef]
- Martin, L.; Engelkamp, H.; Akutsu, H.; Nakatsuji, S.; Yamada, J.; Horton, P.; Hursthouse, M.B. Radical-cation salts of BEDT-TTF with lithium tris(oxalato)metallate(III). Dalton Trans. 2015, 44, 6219–6223. [Google Scholar] [CrossRef] [PubMed]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Coexistence of two donor packing motifs in the stable molecular metal α-pseudo-κ-(BEDT-TTF)4(H3O)[Fe(C2O4)3]·C6H4Br2. CrystEngComm 2011, 13, 2430–2438. [Google Scholar] [CrossRef]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Day, P.; Canadell, E.; Firth, S.; Clark, R.J.H.; Yamada, J.; Nakatsuji, S. Superstructures of donor packing arrangements in a series of molecular charge transfer salts. Chem. Commun. 2004, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Day, P.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Hursthouse, M.B.; McMillan, P.; et al. Metallic molecular crystals containing chiral or racemic guest molecules. CrystEngComm 2007, 9, 865–867. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B.; Harrington, R.W.; Clegg, W. Chiral Radical-Cation Salts of BEDT-TTF Containing a Single Enantiomer of Tris(oxalato)aluminate(III) and -chromate(III). Eur. J. Inorg. Chem. 2015, 1865–1870. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Horton, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H. Chiral conducting salts of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallised from a chiral solvent. J. Mater. Chem. 2010, 20, 2738–2742. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B. Chirality in charge-transfer salts of BEDT-TTF of tris(oxalato)chromate(III). CrystEngComm 2015, 17, 2783–2790. [Google Scholar] [CrossRef]
- Coldea, A.I.; Bangura, A.F.; Singleton, J.; Ardavan, A.; Akutsu-Sato, A.; Akutsu, H.; Turner, S.S.; Day, P. Fermi-surface topology and the effects of intrinsic disorder in a class of charge-transfer salts containing magnetic ions: β′′-(BEDT-TTF)4[(H3O)M(C2O4)3]·Y (M = Ga, Cr, Fe; Y = C5H5N). Phys. Rev. B 2004, 69, 085112. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Mabbs, F.E.; McInnes, E.J.L. New molecular superconductor containing paramagnetic chromium (III) ions. Chem. Commun. 1997, 1367–1368. [Google Scholar] [CrossRef]
- Rosseinsky, M.J.; Kurmoo, M.; Talham, D.R.; Day, P.; Chasseau, D.; Watkin, D. A Novel Conducting Charge-Transfer Salt-(bedt-ttf)3Cl2·2H2O. J. Chem. Soc. Chem. Commun. 1988, 88–90. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, Y.X.; Zhu, D.B. A new organic conductor (BEDT-TTF)5Cl3(H2O)5. Synth. Met. 2001, 120, 671–674. [Google Scholar] [CrossRef]
- Mori, H.; Hirabayashi, I.; Tanaka, S.; Maruyama, Y. Preparation, Crystal and Electronic Structures, and Electrical Resistivity of (BEDT-TTF)3Cl2.5(H5O2). Bull. Chem. Soc. Jpn. 1993, 66, 2156–2159. [Google Scholar] [CrossRef]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Kanehama, R.; Umemiya, M.; Iwahori, F.; Miyasaka, H.; Sugiura, K.; Yamashita, M.; Yokochi, Y.; Ito, H.; Kuroda, S.; Kishida, H.; et al. Novel ET-Coordinated Copper(I) Complexes: Syntheses, Structures, and Physical Properties (ET = BEDT-TTF = Bis(ethylenedithio)tetrathiafulvalene). Inorg. Chem. 2003, 42, 7173–7181. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Inokuchi, H. A BEDT-TTF Complex Including a Magnetic Anion, (BEDT-TTF)3(MnCl4)2. Bull. Chem. Soc. Jpn. 1988, 61, 591–593. [Google Scholar] [CrossRef]
- Shibaeva, R.P.; Lobkovskaya, R.M.; Korotkov, V.E.; Kusch, N.D.; Yagubskii, E.B.; Makova, M.K. ET cation-radical salts with metal complex anions. Synth. Met. 1988, 27, A457–A463. [Google Scholar] [CrossRef]
- Chou, L.; Quijada, M.A.; Clevenger, M.B.; de Oliveira, G.F.; Abboud, K.A.; Tanner, D.B.; Talham, D.R. Dication Salts of the Organic Donor Bis(ethylenedithio)tetrathiafulvalene. Chem. Mater. 1995, 7, 530–534. [Google Scholar] [CrossRef]
- Naito, T.; Inabe, T.; Takeda, K.; Awaga, K.; Akutagawa, T.; Hasegawa, T.; Nakamura, T.; Kakiuchi, T.; Sawa, H.; Yamamoto, T.; et al. β′′-(ET)3(MnCl4)(1,1,2-C2H3Cl3) (ET = bis(ethylenedithio)tetrathiafulvalene); a pressure-sensitive new molecular conductor with localized spins. J. Mater. Chem. 2001, 11, 2221–2227. [Google Scholar] [CrossRef]
- Naito, T.; Inabe, T. Structural, Electrical, and Magnetic Properties of alpha-(ET)7[MnCl4]2·(1,1,2-C2H3Cl3)2 (ET = Bis(ethylenedithio)tetrathiafulvalene). Bull. Chem. Soc. Jpn. 2004, 77, 1987–1995. [Google Scholar] [CrossRef]
- Willett, R.D.; Gómez-García, C.J.; Twamley, B.; Gómez-Coca, S.; Ruiz, E. Exchange coupling mediated by N–H···Cl hydrogen bonds: Experimental and theoretical study of the frustrated magnetic system in bis(o-phenylenediamine)nickel(II) chloride. Inorg. Chem. 2012, 51, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.G. Experimental Inorganic Chemistry; Cambridge University Press: Cambridge, UK, 1954. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]








| CCDC Code | MIII | AI | G | Packing | SG | Elect. Prop. | Ref. |
|---|---|---|---|---|---|---|---|
| ZIGYET | Fe | H3O+ | PhCN | β′′ | C2/c | Tc = 7.0–8.5 K | [6,7,32,33,34] |
| KILFOB/GOC/GUI/HAP | Fe | H3O+ | C5H5N(1−x)/PhCNx | β′′ | C2/c | Tc = F(x) | [33] |
| BEMPEO/QAL | Fe | H3O+ | C5H5N | β′′ | C2/c | TMI = 116 K | [33,35] |
| ECOPIV | Fe | H3O+/NH4+ | PhNO2 | β′′ | C2/c | Tc = 6.2 K | [36] |
| COQNEB | Fe | H3O+ | PhNO2 | β′′ | C2/c | Semicond | [37,38] |
| PONMEL | Fe | H3O+ | PhCl2 | β′′ | C2/c | TMI = 3.0 K, Metal > 1.5 K | [34,39] |
| SAPWEM | Fe | H3O+ | PhBr | β′′ | C2/c | Tc = 4.0 K | [40] |
| UMACEQ | Fe | NH4+ | DMF | β′′ | C2/c | Metal > 4 K | [41] |
| UJOXEX | Fe | H3O+ | PhF | β′′ | C2/c | Tc = 1.0 K | [34,42,43] |
| UJOXAT | Fe | H3O+ | PhCl | β′′ | C2/c | Metal > 0.4 K | [34,42,43,44,45] |
| UJOXIB | Fe | H3O+ | PhF/PhCN | β′′ | C2/c | Tc = 6.0 K | [34,42] |
| UJOXOH | Fe | H3O+ | PhCl2/PhCN | β′′ | C2/c | Tc = 7.2 K | [34] |
| UJOYAU | Fe | H3O+ | PhCl/PhCN | β′′ | C2/c | Tc = 6.0 K | [34,42] |
| UJOYEY | Fe | H3O+ | PhBr/PhCN | β′′ | C2/c | Tc = 4.2 K | [34,42] |
| QAXSIT | Fe | K+ | PhI | β′′ | C2/c | Ea = 64 meV | [43] |
| - | Fe | K+ | PhCl | β′′ | - | Semicond. | [46] |
| - | Fe | Rb+ | C5H5N | β′′ | - | Metal > 4.2 K | [44] |
| JUPGUW01 | Cr | H3O+ | PhCN | β′′ | C2/c | Tc = 5.5–6.0 K | [32,47] |
| MEQZIR | Cr | H3O+ | CH2Cl2 | β′′ | C2/c | TMI = 150 K | [48] |
| ECOPUH | Cr | H3O+/NH4+ | PhNO2 | β′′ | C2/c | Tc = 5.8 K | [36] |
| - | Cr | H3O+ | PhBr | β′′ | C2/c | Tc = 1.5 K | [45] |
| - | Cr | H3O+ | PhCl | β′′ | C2/c | TMI = 130 K | [45] |
| UMACAM | Cr | K+/NH4+ | DMF | β′′ | C2/c | Metal > 4 K | [41] |
| UMACIU | Cr | K+ | DMF | β′′ | C2/c | Metal > 4 K | [41] |
| HUNQIQ | Ga | H3O+ | C5H5N | β′′ | C2/c | Tc ≈ 2 K | [49] |
| HUNQUC | Ga | H3O+ | PhNO2 | β′′ | C2/c | Tc = 7.5 K | [49] |
| HOBROH | Ga | H3O+/K+ | PhBr | β′′ | C2/c | metal > 0.5 K | [50] |
| UDETUU | Ru | H3O+/K+ | PhCN | β′′ | C2/c | Tc = 6.3 K | [51] |
| YUYTUJ | Fe | H3O+ | 2-Cl–Py | β′′ | C2/c | Tc = 4.0 K | [52] |
| YUYVEV | Fe | H3O+ | 2-Br–py | β′′ | C2/c | Tc = 4.3 K | [52] |
| YUYVOF | Fe | H3O+ | 3-Cl–py | β′′ | C2/c | metal > 0.5 K | [52] |
| YUYVUL | Fe | H3O+ | 3-Br-py | β′′ | C2/c | metal > 0.5 K | [52] |
| DUDWOQ | Fe | Li+ + H2O | EtOH | η (α″) | P21/n | Ea = 80 meV | [53] |
| - | Mn | H3O+ | PhBr | β′′ | C2/c | Tc = 2.0 K | [43] |
| CCDC Code | MIII | AI | G | ET Packing | Space Group | Electrical Properties | Ref. |
|---|---|---|---|---|---|---|---|
| UJOXUN | Fe | H3O+ | PhF/PhCN | pseudo-κ | Pbcn | Semiconductor | [34] |
| ZIWNEY | Fe | NH4+ | PhCN | pseudo-κ | Pbcn | Ea = 140 meV | [7,32] |
| ZIWNIC | Fe | K+ | PhCN | pseudo-κ | Pbcn | Ea = 141 meV | [7] |
| JUPGUW | Cr | H3O+ | PhCN | pseudo-κ | Pbcn | Ea = 153 meV | [32,47] |
| QIWMOY | Co | NH4+ | PhCN | pseudo-κ | Pbcn | Ea = 225 meV | [32] |
| QIWMUE | Al | NH4+ | PhCN | pseudo-κ | Pbcn | Ea = 222 meV | [32] |
| UDETOO | Ru | H3O+/K+ | PhCN | pseudo-κ | Pbcn | - | [51] |
| 1 | Mn | K+ | PhCN | pseudo-κ | Pbcn | Ea = 180 meV | this work |
| CCDC Code | MIII | AI | G | ET Packing | Space Group | Electrical Properties | Ref. |
|---|---|---|---|---|---|---|---|
| TANDIX | Fe | H3O+ | PhBr2 | α + κ | P-1 | Metal > 0.4 K | [34,54] |
| HOBRIB | Ga | H3O+/K+ | PhBr2 | α + κ | P-1 | metal > 0.5 K | [50] |
| ARABEA | Fe | NH4+ | PhCOCH3 | α + β′′ | P-1 | No supercond | [55] |
| CILDIL | Fe | NH4+ | R/S-Ph-CH2OHCH3 | α + β′′ | P-1 | TMI = 170 K | [56] |
| NIPTEM | Fe | NH4+ | S-PhCH2OHCH3 | α + β′′ | P-1 | TMI = 150 K | [56] |
| AQUZUH | Ga | NH4+ | PhN(Me)CHO | α + β′′ | P-1 | Semicond | [55] |
| ARABAW | Ga | NH4+ | PhCH2CN | α + β′′ | P-1 | Semicond | [55] |
| CCDC Code | MIII | AI | G | ET Packing | Space Group | Electrical Properties | Ref. |
|---|---|---|---|---|---|---|---|
| BOYTIU | Al | Na+ | CH3NO2 | dimers + mon. | P21 | Ea ≈ 140 meV | [57] |
| XUNXOU01 | Cr | Na+ | CH3NO2 | dimers + mon. | P21 | Ea = 79 meV | [58] |
| XUNXOU | Cr | Na+ | CH3NO2 | dimers + mon. | P212121 | Ea = 80 meV | [58] |
| - | Cr | NH4+ | CH3NO2 | dimers + mon. | P212121 | Ea = 80 meV | [58] |
| DUXNOA | Cr | Na+ | CH2Cl2 | dimers + mon. | P1 | Ea = 69 meV | [22] |
| DUDWUW | Cr | Li+ | EtOH | dimers + mon. | P21/c | Ea = 179 meV | [53] |
| - | Fe | Li+ | EtOH | dimers + mon. | P21/c | Ea = 126 meV | [53] |
| KOGMUG01 | Cr | Na+ | CH3CN | dimers + mon. | P21 | Ea = 79 meV | [59] |
| - | Cr | Na+ | DMF | θ-packing | P1 | Ea = 43 meV | [59] |
| YUCLOZ | Cr | Na+ | EtOH | dimers + mon. | P1 | no data | [59] |
| Compound | 1 | 2 |
|---|---|---|
| Formula | C53H36KMnNO12S32 | C10H10MnCl4OS8 |
| F. Wt. | 1999.97 | 599.45 |
| Space group | Pbcn | Pnna |
| Crystal system | Orthorhombic | Orthorhombic |
| a (Å) | 10.3727 (4) | 12.3724 (9) |
| b (Å) | 19.6588 (8) | 12.3738 (9) |
| c (Å) | 36.2145 (13) | 13.7726 (13) |
| V/Å3 | 7384.7 (5) | 2108.5 (3) |
| Z | 4 | 4 |
| T (K) | 120 | 120 |
| ρcalc/g·cm−3 | 1.798 | 1.856 |
| μ/mm−1 | 1.199 | 1.923 |
| F(000) | 4052 | 1156 |
| R(int) | 0.1380 | 0.1089 |
| θ range (deg) | 2.910–25.053 | 2.958–25.044 |
| Total reflections | 59,518 | 14,081 |
| Unique reflections | 6534 | 1867 |
| Data with I > 2σ (I) | 6534 | 1867 |
| Nvar | 462 | 114 |
| R1 a on I > 2σ (I) | 0.0700 | 0.0509 |
| wR2 b (all) | 0.1729 | 0.1006 |
| GOF c on F2 | 1.011 | 1.080 |
| Δρmax (eÅ−3) | 0.561 | 1.130 |
| Δρmin (eÅ−3) | −0.839 | −0.553 |
| Atoms | Distance (Å) | Atoms | Distance (Å) |
|---|---|---|---|
| S6Ai···S6Bii | 3.563 | S3Aii···S7Bi | 3.294 |
| S8Ai···S8Bii | 3.571 | S5Aii···S5Bi | 3.446 |
| S2Ai···S8Biii | 3.497 | S7Aii···S7Bi | 3.539 |
| S8Ai···S2Biii | 3.564 | - | - |
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Molecule | a | b | c | d | δ | Q |
| 1 | A | 1.392 | 1.723 | 1.7468 | 1.3415 | 0.7363 | 0.85 |
| B | 1.353 | 1.7542 | 1.7565 | 1.337 | 0.8207 | 0.22 | |
| 2 | A | 1.424 | 1.694 | 1.718 | 1.380 | 0.6080 | 1.81 |
| Atoms | Distance (Å) | Atoms | Distance (Å) | Atoms | Distance (Å) |
|---|---|---|---|---|---|
| Cl1-S5A | 3.250 | Cl1ii-S2Aiii | 3.417 | S2Av-S2Avi | 3.412 |
| Cl1-C7A’ | 3.437 | Cl2ii-S2Aiii | 3.446 | S2Av-S6Avi | 3.597 |
| Cl1-S1Ai | 3.353 | Cl2ii-S6Aiv | 3.295 | C7A’v-O1Wvii | 3.147 |
| Compound | Formula | Mn–Cl (Å) | Ref |
|---|---|---|---|
| ECIQEM | β′′-(ET)3MnCl4·TCE | 2.360 | [70] |
| FEWJAT | α-(ET)7[MnCl4]2·TCE | 2.348 | [71] |
| GAMSOC | (ET)3[MnCl4]2 | 2.363 | [67] |
| 2 | (ET)[MnCl4]·H2O | 2.368 | this work |
| Compound | Anode | Cathode | Current | Time |
|---|---|---|---|---|
| (ET)4[KMn(C2O4)3]·PhCN (1) | ET (10 mg) PhCN (10 mL) MeOH (1 mL) | K3[Mn(C2O4)3] (0.1 mmol) 18-crown-6 (90 mg) PhCOOH (0.147 mmol) PhCN (10 mL) MeOH (1.5 mL) | 3 μA | 1 week |
| (ET)[MnCl4] H2O (2) | ET (10 mg) TCE (10 mL) MeOH (1 mL) | K3[Mn(C2O4)3] (0.1 mmol) 18-crown-6 (90 mg) TCE (10 mL) MeOH (1 mL) | 2 μA 4 μA 5 μA | 3 weeks 1 week 1 week |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmansour, S.; Sánchez‐Máez, Y.; Gómez‐García, C.J. Mn‐Containing Paramagnetic Conductors with Bis(ethylenedithio)tetrathiafulvalene (BEDT‐TTF). Magnetochemistry 2017, 3, 7. https://doi.org/10.3390/magnetochemistry3010007
Benmansour S, Sánchez‐Máez Y, Gómez‐García CJ. Mn‐Containing Paramagnetic Conductors with Bis(ethylenedithio)tetrathiafulvalene (BEDT‐TTF). Magnetochemistry. 2017; 3(1):7. https://doi.org/10.3390/magnetochemistry3010007
Chicago/Turabian StyleBenmansour, Samia, Yolanda Sánchez‐Máez, and Carlos J. Gómez‐García. 2017. "Mn‐Containing Paramagnetic Conductors with Bis(ethylenedithio)tetrathiafulvalene (BEDT‐TTF)" Magnetochemistry 3, no. 1: 7. https://doi.org/10.3390/magnetochemistry3010007
APA StyleBenmansour, S., Sánchez‐Máez, Y., & Gómez‐García, C. J. (2017). Mn‐Containing Paramagnetic Conductors with Bis(ethylenedithio)tetrathiafulvalene (BEDT‐TTF). Magnetochemistry, 3(1), 7. https://doi.org/10.3390/magnetochemistry3010007