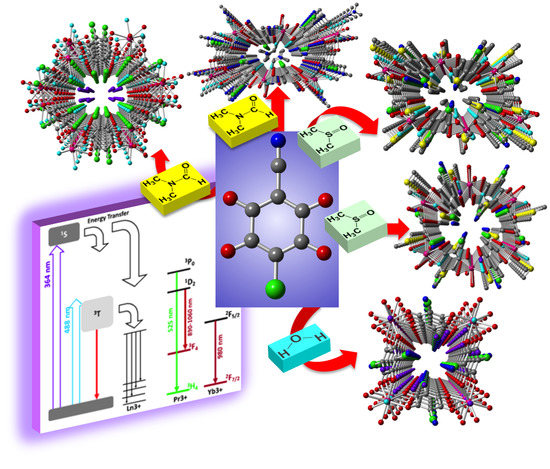
Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses of the Complexes
2.2. Description of the Structures
2.3. Magnetic Properties
2.4. Luminescent Properties
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of [Ce2(C6O4Cl(CN))3(DMF)6]·2H2O (1)
3.3. Synthesis of [Pr2(C6O4(CN)Cl)3(DMF)6] (2)
3.4. Synthesis of [Pr2(C6O4(CN)Cl)3(DMSO)6] (3)
3.5. Synthesis of [Yb2(C6O4(CN)Cl)3(DMSO)4]·2H2O (4)
3.6. Synthesis of (H3O)[Dy(C6O4Cl(CN))2(H2O) ]·4H2O (5)
3.7. Magnetic Measurements
3.8. Luminesce Measurements
3.9. Crystallographic Data Collection and Refinement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Barea, E.; Montoro, C.; Navarro, J.A. Toxic gas removal-metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal-organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Dinca, M. Metal-Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Fordham, S.; Wang, X.; Bosch, M.; Zhou, H. Lanthanide Metal-Organic Frameworks: Syntheses, Properties, and Potential Applications. In Structure and Bonding; Springer: Berlin, Germany, 2015; Volume 163, pp. 1–27. [Google Scholar]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, W.; Bouwman, E. One-step growth of lanthanoid metal-organic framework (MOF) films under solvothermal conditions for temperature sensing. Chem. Commun. (Camb.) 2016, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, W.; Wang, R.; Ren, C.; Li, Q.; Fan, Y.; Liu, B.; Liu, P.; Wang, Y. Effect of Coordinated Solvent Molecules on Metal Coordination Sphere and Solvent-Induced Transformations. Cryst. Growth Des. 2017, 17, 517–526. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Li, X.; Fu, Z.; Su, Y.; Xu, G. Porous Cadmium(II) Anionic Metal-Organic Frameworks Based on Aromatic Tricarboxylate Ligands: Encapsulation of Protonated Flexible Bis(2-methylimidazolyl) Ligands and Proton Conductivity. Cryst. Growth Des. 2015, 15, 4543–4548. [Google Scholar] [CrossRef]
- Sun, L.; Qi, Y.; Che, Y.; Batten, S.R.; Zheng, J. Three Unprecedented Entangled Metal-Organic Frameworks: Self-Penetration and Hydrothermal in Situ Ligand Formation. Cryst. Growth Des. 2009, 9, 2995–2998. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.C. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Sessini, E.; Marchio, L.; Loche, D.; Serpe, A.; Deplano, P.; Concas, G.; Pop, F.; Avarvari, N.; et al. Halogen-bonding in a new family of tris(haloanilato)metallate(III) magnetic molecular building blocks. Dalton Trans. 2014, 43, 7006–7019. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Gómez-Claramunt, P.; Vallés-García, C.; Mínguez Espallargas, G.; Gómez García, C.J. Key Role of the Cation in the Crystallization of Chiral Tris(Anilato)Metalate Magnetic Anions. Cryst. Growth Des. 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Grannas, M.J.; Hudson, T.A.; Hughes, S.A.; Pranoto, N.H.; Robson, R. Synthesis, structure and host-guest properties of (Et4N)2[SnIVCaII(chloranilate)4], a new type of robust microporous coordination polymer with a 2D square grid structure. Dalton Trans. 2011, 40, 12242–12247. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benmansour, S.; Mínguez Espallargas, G.; Clemente-León, M.; Abhervé, A.; Gómez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A Family of Layered Chiral Porous Magnets Exhibiting Tunable Ordering Temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Vallés-García, C.; Gómez-Claramunt, P.; Mínguez Espallargas, G.; Gómez-García, C.J. 2D and 3D Anilato-Based Heterometallic M(I)M(III) Lattices: The Missing Link. Inorg. Chem. 2015, 54, 5410–5418. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Gómez-García, C.J. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers 2016, 8, 89. [Google Scholar] [CrossRef]
- Benmansour, S.; Abhervé, A.; Gómez-Claramunt, P.; Vallés-García, C.; Gómez-García, C.J. Nanosheets of Two-Dimensional Magnetic and Conducting Fe(II)/Fe(III) Mixed-Valence Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 26210–26218. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Coleiro, J.; Ha, K.; Hoskins, B.F.; Orchard, S.D.; Robson, R. Dihydroxybenzoquinone and chloranilic acid derivatives of rare earth metals. J. Chem. Soc. Dalton Trans. 2002, 1586–1594. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Coleiro, J.; Hoskins, B.F.; Robson, R. Gas hydrate-like pentagonal dodecahedral M2(H2O)18 cages (M = lanthanide or Y) in 2,5-dihydroxybenzoquinone-derived coordination polymers. Chem. Commun. 1996, 603–604. [Google Scholar] [CrossRef]
- López-Martínez, G. Multifunctionality in Molecular Materials Based on Anilato-Type Ligands. Ph.D. Thesis, University of Valencia, Valencia, Spain, June 2017. [Google Scholar]
- Christian, R. Complexes with substituted 2,5-dihydroxy-p-benzoquinones: The inclusion compounds [Y(H2O)3]2 (C6Cl2O4)3·6.6H2O and [Y(H2O)3]2 (C6Br2O4)3·6H2O. Mater. Res. Bull. 1987, 22, 1483–1491. [Google Scholar]
- Riley, P.E.; Haddad, S.F.; Raymond, K.N. Preparation of praseodymium(III) chloranilate and the crystal structures of Pr2(C6Cl2O4)3·8C2H5OH and Na3[C6H2O(OH)(SO3)2] H2O. Inorg. Chem. 1983, 22, 3090–3096. [Google Scholar] [CrossRef]
- Benmansour, S.; Pérez-Herráez, I.; López-Martínez, G.; Gómez García, C.J. Solvent-modulated structures in anilato-based 2D coordination polymers. Polyhedron 2017, 135, 17–25. [Google Scholar] [CrossRef]
- Benmansour, S.; López-Martínez, G.; Canet-Ferrer, J.; Gómez-García, C.J. A Family of Lanthanoid Dimers with Nitroanilato Bridges. Magnetochemistry 2016, 2, 32. [Google Scholar] [CrossRef]
- Benmansour, S.; Hernández-Paredes, A.; Gómez-García, C.J. Size-dependence of the structure in a series of lanthanoid-anilato 2D lattices. J. Coord. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Marchio, L.; Loche, D.; Caneschi, A.; Serpe, A.; Delano, P.; Avarvari, N.; Mercuri, M.L. Switching-on luminescence in anilate-based molecular materials. Dalton Trans. 2015, 44, 15786–15802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehwoldt, R.E.; Chasen, B.; Li, J. 2-Chloro-5-Cyano-3,6-Dihydroxybenzoquinone, a New Analytical Reagent for the Spectrophotometric Determination of Calcium(II). Anal. Chem. 1966, 38, 1018–1019. [Google Scholar] [CrossRef]
- Diaz-Torres, R.; Alvarez, S. Coordinating ability of anions and solvents towards transition metals and lanthanides. Dalton Trans. 2011, 40, 10742–10750. [Google Scholar] [CrossRef] [PubMed]
- Imaz, I.; Mouchaham, G.; Roques, N.; Brandès, S.; Sutter, J. Tetradihydrobenzoquinonate and Tetrachloranilate Zr(IV) Complexes: Single-Crystal-to-Single-Crystal Phase Transition and Open-Framework Behavior for K4Zr(DBQ)4. Inorg. Chem. 2013, 52, 11237–11243. [Google Scholar] [CrossRef] [PubMed]
- Mouchaham, G.; Roques, N.; Duhayon, C.; Imaz, I.; Sutter, J. Extended H-bond networks based on guanidinium H-donors and [Zr(A)4]4− H-acceptor units: Modulation of the assemblage and guest accessible volume by chemical design (A = oxalate, dihydrobenzoquinonate, chloranilate). New J. Chem. 2013, 37, 3476–3487. [Google Scholar] [CrossRef]
- Sorace, L.; Gatteschi, D. Electronic Structure and Magnetic Properties of Lanthanide Molecular Complexes. In Lanthanides and Actinides in Molecular Magnetism; Layfield, R.A., Murugesu, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, pp. 1–25. [Google Scholar]
- Sontakke, A.D.; Ueda, J.; Katayama, Y.; Zhuang, Y.; Dorenbos, P. Role of electron transfer in Ce3+ sensitized Yb3+ luminescence in borate glass. J. Appl. Phys. 2015, 117, 013105. [Google Scholar] [CrossRef] [Green Version]
- Kristianpoller, N.; Shmilevich, A.; Weiss, D.; Chen, R.; Khaidukov, N. Luminescence of LiKYF5:Pr3 crystals. Radiat. Meas. 2001, 33, 637–640. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
















| Ln(III) | Compound | Phase | Cavity | Ref. |
|---|---|---|---|---|
| La | [La2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Ce | [Ce2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Pr | [Pr2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Nd | [Nd2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Sm | [Sm2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Eu | [Eu2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Gd | [Gd2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Tb | [Tb2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Dy | [Dy2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Ho | [Ho2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Er | [Er2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Tm | [Tm2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [33] |
| Yb | [Yb2(dhbq)3(H2O)6]·18H2O | I | hex-regular | [31] |
| Ln(III) | Compound | Phase | Cavity | Ref. |
|---|---|---|---|---|
| La | [La2(CA)3(H2O)6]·14H2O a | I | hex-4E-2F | [31] |
| Ce | [Ce2(CA)3(H2O)6]·14H2O b | I | hex-4E-2F | [31] |
| Pr | [Pr2(CA)3(H2O)6]·14H2O | I | hex-4E-2F | [31] |
| Nd | [Nd2(CA)3(H2O)6]·14H2O c | I | hex-4E-2F | [31] |
| Sm | [Sm2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [33] |
| Eu | [Eu2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [31] |
| Gd | [Gd2(CA)3(H2O)6]·12H2O d | II | hex-4E-2F | [31] |
| Tb | [Tb2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [31] |
| Dy | [Dy2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [33] |
| Ho | [Ho2(CA)3(H2O)6]·12H2O | II | hex-4E-2F | [33] |
| Er | [Er2(CA)3(H2O)6]·10H2O | III | hex-2E-4F | [33] |
| Tm | [Tm2(CA)3(H2O)6]·8H2O | IV | rect-4E-2F | [33] |
| Yb | [Yb2(CA)3(H2O)6]·8H2Oe | IV | rect-4E-2F | [31] |
| Ln(III) | D = H2O/G = H2O | Phase | Cavity | Ref. |
|---|---|---|---|---|
| La | [La2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Ce | [Ce2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Pr | [Pr2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Nd | [Nd2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Sm | [Sm2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Eu | [Eu2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Gd | [Gd2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Tb | [Tb2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Dy | [Dy2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Ho | [Ho2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [33] |
| Er | [Er2(BA)3(H2O)6]·12H2O | I | hex-4E-2F | [36] |
| Tm | [Tm2(BA)3(H2O)6]·8H2O | II | rect-4E-2F | [33] |
| Yb | [Yb2(BA)3(H2O)6]·8H2O | II | rect-4E-2F | [33] |
| Ln(III) | D = H2O/G = H2O | Phase | Ref. |
|---|---|---|---|
| Sm | [Sm2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Eu | [Eu2(NA)3(H2O)10]·6H2O | dimer | [33] |
| Gd | [Gd2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Tb | [Tb2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Dy | [Dy2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Ho | [Ho2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Er | [Er2(NA)3(H2O)10]·6H2O | dimer | [37] |
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | C39H46Cl3 N9O20Ce2 | C39H42Cl3 N9O18Pr2 | C33H36Cl3 N3O18S6Pr2 | C29H28Cl3 N3O18S4Yb2 | C14H13Cl2N2 O14Dy |
| F. Wt. | 1347.42 | 1312.98 | 1343.21 | 1287.24 | 666.66 |
| Space group (#) | C2/c (15) | P21/n (14) | P21/n (14) | P-1 (2) | P2/n (13) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic | Monoclinic |
| a (Å) | 13.8141(3) | 10.6585(10) | 9.6262(3) | 9.9354 | 11.9237(4) |
| b (Å) | 23.3672(4) | 13.7163(10) | 16.3853(4) | 10.6195 | 14.0496(8) |
| c (Å) | 17.9855(4) | 18.3196(14) | 15.3240(4) | 10.7752 | 12.2263(5) |
| α (°) | 90 | 90 | 90 | 112.010 | 90 |
| β (°) | 98.983(2) | 98.863 | 91.765 | 93.135 | 90.254(4) |
| γ (°) | 90 | 90 | 90 | 95.19 | 90 |
| V/Å3 | 5734.5(2) | 2646.3(4) | 2415.88(11) | 1044.39(7) | 2048.17(16) |
| Z | 4 | 2 | 2 | 1 | 1 |
| T (K) | 120 | 120 | 120 | 120 | 120 |
| ρcalc/g cm−3 | 1.638 | 1.633 | 1.797 | 2.021 | 1.189 |
| μ/mm−1 | 1.921 | 2.045 | 2.488 | 4.916 | 2.009 |
| F(000) | 2796 | 1280 | 1256 | 606 | 700 |
| R(int) | 0.0314 | 0.0898 | 0.1180 | 0.0594 | 0.0623 |
| θ range (deg) | 3.31–25.08 | 2.81–25.14 | 2.76–25.07 | 3.05–25.03 | 3.33–25.05 |
| Total reflections | 19,380 | 35,858 | 38,300 | 10,448 | 14,772 |
| Unique reflections | 5071 | 4705 | 4278 | 3180 | 3604 |
| Data with I > 2σ (I) | 4450 | 3541 | 3154 | 3207 | 3264 |
| Nvar | 342 | 327 | 311 | 289 | 186 |
| R1 a on I > 2σ (I) | 0.0599 | 0.0442 | 0.0606 | 0.0394 | 0.0839 |
| wR2 b (all) | 0.1773 | 0.1139 | 0.1497 | 0.1010 | 0.2384 |
| GOF c on F2 | 1.089 | 1.052 | 1.067 | 1.071 | 1.113 |
| Δρmax (eÅ−3) | 1.810 | 1,616 | 1.419 | 1.775 | 4.534 |
| Δρmin (eÅ−3) | −1.980 | −0,865 | −1.004 | −1.100 | −1.419 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Claramunt, P.; Benmansour, S.; Hernández-Paredes, A.; Cerezo-Navarrete, C.; Rodríguez-Fernández, C.; Canet-Ferrer, J.; Cantarero, A.; Gómez-García, C.J. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry 2018, 4, 6. https://doi.org/10.3390/magnetochemistry4010006
Gómez-Claramunt P, Benmansour S, Hernández-Paredes A, Cerezo-Navarrete C, Rodríguez-Fernández C, Canet-Ferrer J, Cantarero A, Gómez-García CJ. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry. 2018; 4(1):6. https://doi.org/10.3390/magnetochemistry4010006
Chicago/Turabian StyleGómez-Claramunt, Patricia, Samia Benmansour, Antonio Hernández-Paredes, Christian Cerezo-Navarrete, Carlos Rodríguez-Fernández, Josep Canet-Ferrer, Andrés Cantarero, and Carlos J. Gómez-García. 2018. "Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand" Magnetochemistry 4, no. 1: 6. https://doi.org/10.3390/magnetochemistry4010006
APA StyleGómez-Claramunt, P., Benmansour, S., Hernández-Paredes, A., Cerezo-Navarrete, C., Rodríguez-Fernández, C., Canet-Ferrer, J., Cantarero, A., & Gómez-García, C. J. (2018). Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry, 4(1), 6. https://doi.org/10.3390/magnetochemistry4010006