Chloranilato-Based Layered Ferrimagnets with Solvent-Dependent Ordering Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses of the Complexes
2.2. FT-IR Spectra
2.3. Thermogravimetric Analysis
2.4. Structures of Compounds 1–6
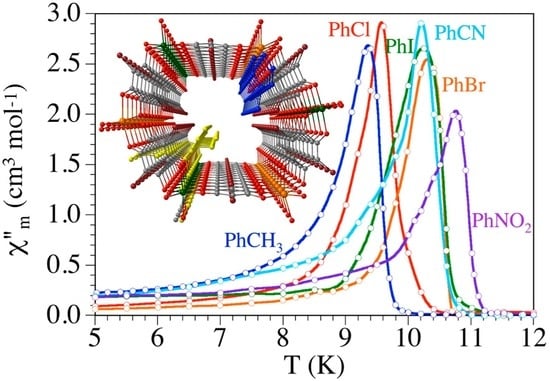
2.5. Magnetic Properties
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of (NBu4)[MnCr(C6O4Cl2)3]·C6H5Cl (1)
3.3. Synthesis of (NBu4)[MnCr(C6O4Cl2)3]·1.5C6H5Br (2)
3.4. Synthesis of (NBu4)[MnCr(C6O4Cl2)3]·C6H5I (3)
3.5. Synthesis of (NBu4)[MnCr(C6O4Cl2)3]·C6H5CH3 (4)
3.6. Synthesis of (NBu4)[MnCr(C6O4Cl2)3]·2C6H5CN (5)
3.7. Synthesis of (NBu4)[MnCr(C6O4Cl2)3]·2C6H5NO2 (6)
3.8. Magnetic Measurements
3.9. X-ray Powder Diffraction
3.10. Physical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, J.S.; Gatteschi, D. Molecule-based magnets. Chem. Soc. Rev. 2011, 40, 3065–3066. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, M.; Bleuzen, A.; Marvaud, V.; Vaissermann, J.; Seuleiman, M.; Desplanches, C.; Scuiller, A.; Train, C.; Garde, R.; Gelly, G.; et al. Molecules to build solids: High TC molecule-based magnets by design and recent revival of cyano complexes chemistry. Coord. Chem. Rev. 1999, 190, 1023–1047. [Google Scholar] [CrossRef]
- Ohba, M.; Ōkawa, H. Synthesis and magnetism of multi-dimensional cyanide-bridged bimetallic assemblies. Coord. Chem. Rev. 2000, 198, 313–328. [Google Scholar] [CrossRef]
- Culp, J.T.; Park, J.H.; Frye, F.; Huh, Y.D.; Meisel, M.W.; Talham, D.R. Magnetism of metal cyanide networks assembled at interfaces. Coord. Chem. Rev. 2005, 249, 2642–2648. [Google Scholar] [CrossRef]
- Weihe, H.; Güdel, H.U. Magnetic Exchange Across the Cyanide Bridge. Comments Inorg. Chem. 2000, 22, 75–103. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodríguez-Fortea, A.; Alvarez, S.; Verdaguer, M. Is it possible to get high TC magnets with Prussian blue analogues? A theoretical prospect. Chem. Eur. J. 2005, 11, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Photoinduced Magnetization of a Cobalt-Iron Cyanide. Science 1996, 272, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Sato, O. Switchable molecular magnets. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamoto, N.; Ohkoshi, S.; Sato, O.; Hashimoto, K. Control of Charge-Transfer-Induced Spin Transition Temperature on Cobalt-Iron Prussian Blue Analogues. Inorg. Chem. 2002, 41, 678–684. [Google Scholar] [CrossRef]
- Miyasaka, H.; Julve, M.; Yamashita, M.; Clérac, R. Slow Dynamics of the Magnetization in One-Dimensional Coordination Polymers: Single-Chain Magnets. Inorg. Chem. 2009, 48, 3420–3437. [Google Scholar] [CrossRef] [PubMed]
- Funck, K.E.; Hilfiger, M.G.; Berlinguette, C.P.; Shatruk, M.; Wernsdorfer, W.; Dunbar, K.R. Trigonal-Bipyramidal Metal Cyanide Complexes: A Versatile Platform for the Systematic Assessment of the Magnetic Properties of Prussian Blue Materials. Inorg. Chem. 2009, 48, 3438–3452. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, S.; Mallah, T.; Ouahes, R.; Veillet, P.; Verdaguer, M.; Ouah, R. A room-temperature organometallic magnet based on Prussian blue. Nature 1995, 378, 701–703. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Okawa, H. Design of metal-complex magnets. Syntheses and magnetic properties of mixed-metal assemblies {NBu4[MCr(ox)3]}x (NBu4+ = tetra(n-butyl)ammonium ion; ox2− = oxalate ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Mathoniere, C.; Nuttall, C.J.; Carling, S.G.; Day, P. Ferrimagnetic Mixed-Valency and Mixed-Metal Tris(oxalato)iron(III) Compounds: Synthesis, Structure, and Magnetism. Inorg. Chem. 1996, 35, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Clement, R.; Decurtins, S.; Gruselle, M.; Train, C. Polyfunctional two-(2D) and three-(3D) dimensional oxalate bridged bimetallic magnets. Monatshefte Chem. 2003, 134, 117–135. [Google Scholar] [CrossRef]
- Benmansour, S.; Cerezo-Navarrete, C.; Canet-Ferrer, J.; Muñoz-Matutano, G.; Martínez-Pastor, J.; Gómez-García, C.J. A fluorescent layered oxalato-based canted antiferromagnet. Dalton Trans. 2018, 47, 11909–11916. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; Galán-Mascarós, J.R.; Gómez-Garcia, C.J. Intercalation of decamethylferrocenium cations in bimetallic oxalate-bridged two-dimensional magnets. Chem. Commun. 1997, 1727–1728. [Google Scholar] [Green Version]
- Coronado, E.; Clemente-León, M.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Martínez-Ferrero, E. Design of molecular materials combining magnetic, electrical and optical properties. J. Chem. Soc. Dalton Trans. 2000, 3955–3961. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Ensling, J.; Gütlich, P. Hybrid molecular magnets obtained by insertion of decamethyl-metallocenium cations into layered, bimetallic oxalate complexes: [ZIIICp*2][MIIMIII(ox)3](ZIII = Co, Fe; MIII = Cr, Fe; MII = Mn, Fe, Co, Cu, Zn; ox = oxalate; Cp* = pentamethylcyclopentadienyl). Chem. Eur. J. 2000, 6, 552–563. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Agudo, J.M. Increasing the coercivity in layered molecular-based magnets A[MIIMIII(ox)3] (MII = Mn, Fe, Co, Ni, Cu; MIII = Cr, Fe; ox = oxalate; A = organic or organometallic cation). Adv. Mater. 1999, 11, 558–561. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Agudo, J.M.; Martínez-Ferrero, E.; Waerenborgh, J.C.; Almeida, M. Layered molecule-based magnets formed by decamethylmetallocenium cations and two-dimensional bimetallic complexes [MIIRuIII(ox)3]−(MII = Mn, Fe, Co, Cu and Zn; ox = oxalate). J. Solid State Chem. 2001, 159, 391–402. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benmansour, S.; Mínguez Espallargas, G.; Clemente-León, M.; Abhervé, A.; Gómez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A Family of Layered Chiral Porous Magnets Exhibiting Tunable Ordering Temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochem 2017, 3, 17. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Halis, S.; Inge, A.K.; Dehning, N.; Weyrich, T.; Reinsch, H.; Stock, N. Dihydroxybenzoquinone as Linker for the Synthesis of Permanently Porous Aluminum Metal–Organic Frameworks. Inorg. Chem. 2016, 55, 7425–7431. [Google Scholar] [CrossRef]
- Jeon, I.R.; Negru, B.; Van Duyne, R.P.; Harris, T.D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef]
- Palacios-Corella, M.; Fernández-Espejo, A.; Bazaga-García, M.; Losilla, E.R.; Cabeza, A.; Clemente-León, M.; Coronado, E. Influence of Proton Conducting Cations on the Structure and Properties of 2D Anilate-Based Magnets. Inorg. Chem. 2017, 56, 13865–13877. [Google Scholar] [CrossRef]
- Riley, P.E.; Haddad, S.F.; Raymond, K.N. Preparation of praseodymium(III) chloranilate and the crystal structures of Pr2(C6Cl2O4)3.8C2H5OH and Na3[C6H2O(OH)(SO3)2].H2O. Inorg. Chem. 1983, 22, 3090–3096. [Google Scholar] [CrossRef]
- Christian, R. Complexes with substituted 2,5-dihydroxy-p-benzoquinones: The inclusion compounds [Y(H2O)3]2(C6Cl2O4)3·6.6H2O and [Y(H2O)3]2(C6Br2O4)3·6H2O. Mater. Res. Bull. 1987, 22, 1483–1491. [Google Scholar]
- Abrahams, B.F.; Coleiro, J.; Hoskins, B.F.; Robson, R. Gas hydrate-like pentagonal dodecahedral M2(H2O)18 cages (M = lanthanide or Y) in 2,5-dihydroxybenzoquinone-derived coordination polymers. Chem. Commun. 1996, 603–604. [Google Scholar] [CrossRef]
- Coleiro, J.; Ha, K.; Orchard, S.D.; Robson, R.; Abrahams, B.F.; Hoskins, B.F. Dihydroxybenzoquinone and chloranilic acid derivatives of rare earth metals. J. Chem. Soc. Dalton Trans. 2002, 1586–1594. [Google Scholar]
- Benmansour, S.; Pérez-Herráez, I.; López-Martínez, G.; Gómez-García, C.J. Solvent-modulated structures in anilato-based 2D coordination polymers. Polyhedron 2017, 135, 17–25. [Google Scholar] [CrossRef]
- Benmansour, S.; Pérez-Herráez, I.; Cerezo-Navarrete, C.; López-Martínez, G.; Martínez Hernández, C.; Gómez-García, C.J. Solvent-modulation of the structure and dimensionality in lanthanoid-anilato coordination polymers. Dalton Trans. 2018, 47, 6729–6741. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Hernández-Paredes, A.; Gómez-García, C.J. Effect of the lanthanoid-size on the structure of a series of lanthanoid-anilato 2-D lattices. J. Coord. Chem. 2018, 71, 845–863. [Google Scholar] [CrossRef]
- Gómez-Claramunt, P.; Benmansour, S.; Hernández-Paredes, A.; Cerezo-Navarrete, C.; Rodríguez-Fernández, C.; Canet-Ferrer, J.; Cantarero, A.; Gómez-García, C.J. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochem 2018, 4, 6. [Google Scholar] [Green Version]
- Benmansour, S.; Hernández-Paredes, A.; Gómez-García, C.J. Two Dimensional Magnetic Coordination Polymers Formed by Lanthanoids and Chlorocyananilato. Magnetochem 2018, 4, 58. [Google Scholar] [CrossRef]
- Ashoka Sahadevan, S.; Monni, N.; Abhervé, A.; Marongiu, D.; Sarritzu, V.; Sestu, N.; Saba, M.; Mura, A.; Bongiovanni, G.; Cannas, C.; et al. Nanosheets of Two-Dimensional Neutral Coordination Polymers Based on Near-Infrared-Emitting Lanthanides and a Chlorocyananilate Ligand. Chem. Mater. 2018, 30, 6575–6586. [Google Scholar] [CrossRef]
- Gómez-Claramunt, P. Anilato-Based Multifunctional Molecular Materials. Ph.D. Thesis, University of Valencia, Valencia, Spain, 2018. [Google Scholar]
- Abhervé, A.; Verneret, M.; Clemente-León, M.; Coronado, E.; Gómez-García, C.J. One-Dimensional and Two-Dimensional Anilate-Based Magnets with Inserted Spin-Crossover Complexes. Inorg. Chem. 2014, 53, 12014–12026. [Google Scholar]
- Abhervé, A.; Mañas-Valero, S.; Clemente-León, M.; Coronado, E. Graphene related magnetic materials: Micromechanical exfoliation of 2D layered magnets based on bimetallic anilate complexes with inserted [FeIII(acac2-trien)]+ and [FeIII(sal2-trien)]+ molecules. Chem. Sci. 2015, 6, 4665–4673. [Google Scholar] [CrossRef]
- Benmansour, S.; Abhervé, A.; Gómez-Claramunt, P.; Vallés-García, C.; Gómez-García, C.J. Nanosheets of Two-Dimensional Magnetic and Conducting Fe(II)/Fe(III) Mixed-Valence Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 26210–26218. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, S.A.; Abhervé, A.; Monni, N.; Sáenz, D.P.; Galán-Mascarós, J.R.; Waerenborgh, J.C.; Vieira, B.J.C.; Auban-Senzier, P.; Pillet, S.; Bendeif, E.; et al. Conducting Anilate-Based Mixed-Valence Fe(II)Fe(III) Coordination Polymer: Small-Polaron Hopping Model for Oxalate-Type Fe(II)Fe(III) 2D Networks. J. Am. Chem. Soc. 2018, 140, 12611–12621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Hernández, C.; Benmansour, S.; Gómez-García, C.J. Modulation of the ordering temperature in anilato-based magnets. Polyhedron 2019, in press. [Google Scholar] [CrossRef]
- PANalytical X’Pert HighScore Plus; PANalytical l. V.: Almelo, The Netherlands, 2012.
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]









| CCDC | Formula | Packing | Space Group | Tc (K) | Hcoer (mT) a | Ref. |
|---|---|---|---|---|---|---|
| MIRFEA | [(H3O)(phz)3][MnCr(C6O4Cl2)3(H2O)] b | eclipsed | P3 | 5.5 | 19.4 | [23] |
| MIRFIE | [(H3O)(phz)3][MnCr(C6O4Br2)3]·2H2O·2CH3COCH3 | eclipsed | P-31m | 6.3 | 34.0 | [23] |
| MIRFOK | [(H3O)(phz)3][MnFe(C6O4Br2)3]·H2O | eclipsed | P-31m | - | - | [23] |
| MIRFUQ | (NBu4)[MnCr(C6O4Cl2)3] | alternated | C2/c | 5.5 | 11.8 | [23] |
| HOWHAE | [Fe(sal2-trien)][MnCr(C6O4Cl2)3]·0.5CH2Cl2·CH3OH·0.5H2O·5CH3CN | alternated | C2221 | 10.0 | 35 | [40] |
| HOWHEI | [Fe(4-OH-sal2-trien)][MnCr(C6O4Cl2)3]·G | alternated | P6122 | 10.4 | 87 | [40] |
| HOWHIM | [Fe(sal2-epe)][MnCr(C6O4Br2)3]·4CH3CN | alternated | P21/c | 10.2 | 10 | [40] |
| HOWHOS | [Fe(5-Cl-sal2-trien)][MnCr(C6O4Br2)3]·CH2Cl2·CH3OH·4H2O·1.5CH3CN | alternated | P21/c | 9.8 | 66 | |
| MUMKUC | [Fe(acac2-trien)][MnCr(C6O4Cl2)3]·2CH3CN | alternated | C2/c | 10.8 | 65 | [41] |
| MUMLAJ | [Fe(acac2-trien)][MnCr(C6O4Br2)3]·2CH3CN | alternated | C2/c | 11.1 | 77 | [41] |
| MUMLEN | [Ga(acac2-trien)][MnCr(C6O4Br2)3]·2CH3CN | alternated | C2/c | 11.6 | 72 | [41] |
| SEPLAD | (Me2NH2)[MnCr(C6O4Br2)3]·2H2O | alternated | P-31c | 7.9 | 90 | [28] |
| SEPLEH | (Et2NH2)[MnCr(C6O4Br2)3] | alternated | P-31c | 8.9 | 100 | [28] |
| SEQCID | (Et3NH)[MnCr(C6O4Cl2)3] | alternated | P-31c | 8.0 | 150 | [28] |
| SEPROX | (Et(i-Pr)2NH)[MnCr(C6O4Br2)3] | alternated | P-31c | 9.0 | 4 c | [28] |
| 1910770 | (NBu4)[MnCr(C6O4Br2)3]·C6H5Br·0.5H2O | eclipsed | P21 | 9.5 | 33 | [39] |
| QEFPOJ | [(H3O)(phz)3][FeFe(C6O4Cl2)3]·12H2O d | eclipsed | P-31m | 2.4 | 1.0 | [42] |
| QEFPID | [(H3O)(phz)3][FeFe(C6O4Br2)3]·12H2O d | eclipsed | P-31m | 2.1 | 1.0 | [42] |
| NIHJEW01 | [C(N2H3)3][FeFe(C6O4(CN)Cl)3]·29H2O d | eclipsed | P3 | 4.0 | 6 | [43] |
| 1909314 | (NBu4)[MnCr(C6O4Cl2)3(C6H5CHO)]·C6H6 b | eclipsed | P21 | 7.0 | 7.6 | [44] |
| 1909315 | (NBu4)[MnCr(C6O4Br2)3(C6H5CHO)]·C6H6 b | eclipsed | P21 | 6.7 | 10 | [44] |
| 1909316 | (NBu4)[MnCr(C6O4Cl2)3(C6H5CHO)]·C6H5CHO b | eclipsed | P21 | 6.8 | 5.0 | [44] |
| 1909317 | (NBu4)[MnCr(C6O4Br2)3(C6H5CHO)]·C6H5CHO b | eclipsed | P21 | 6.7 | 20 | [44] |
| Band | 1 (C6H5Cl) | 2 (C6H5Br) | 3 (C6H5I) | 4 (C6H5CN) | 5 (C6H5CH3) | 6 (C6H5NO2) |
|---|---|---|---|---|---|---|
| ν(C-H) | 2959 2929 2873 | 2959 2929 2873 | 2960 2928 2873 | 2962 2931 2873 | 2960 2928 2873 | 2963 2931 2873 |
| ν(CN) 1 | - | - | - | 2227 | - | - |
| ν(C=O) | 1609 | 1606 | 1607 | 1607 | 1607 | 1606 |
| ν(N-O)as 1 | - | - | - | - | - | 1520 |
| ν(C=C) 1 | 1506 | 1506 * | 1505 | 1507 * | 1505 * | 1505 |
| ν(C=C) + ν(C-O) | 1495 | 1496 | 1496 | 1497 | 1496 | 1495 |
| δ(C-H) | 1383 | 1382 | 1383 | 1383 * | 1383 * | 1383 |
| ν(C-C) + ν(C-O) | 1360 | 1362 | 1361 | 1360 | 1359 | 1361 |
| ν(N-O)s 1 | - | - | - | - | - | 1344 * |
| ν(C-N) 1 | - | - | - | - | - | 877 |
| δ(C-X) | 860 | 858 | 858 | 858 | 858 | 857 |
| ν(C-Cl) 1 | 740 | - | - | - | - | - |
| δ(C-H) 1 | 700 684 | 737 682 | 730 685 | 734 687 | 730 693 | 705 683 |
| ρ(C-X) | 577 | 576 | 576 | 577 | 576 | 577 |
| Compound | Temperature Range (°C) | Experimental Weight Loss (%) | Solvent | Calculated Weight Loss |
|---|---|---|---|---|
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5Cl (1) | 30–200 | 10.8 | 1 C6H5Cl | 10.4 |
| (NBu4)[MnCr(C6O4Cl2)3]·1.5C6H5Br (2) | 30–250 | 22.0 | 1.5 C6H5Br | 19.5 |
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5I (3) | 30–200 | 16.6 | 1 C6H5I | 17.4 |
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5CH3 (4) | 30–290 | 9.7 | 1 C6H5CH3 | 8.7 |
| (NBu4)[MnCr(C6O4Cl2)3]·2C6H5CN (5) | 30–250 | 16.8 | 2 C6H5CN | 17.5 |
| (NBu4)[MnCr(C6O4Cl2)3]·2C6H5NO2 (6) | 30–220 | 20.7 | 2 C6H5NO2 | 20.2 |
| Compound | a (Å) | b (Å) | c (Å) | (°) | Volume (Å3) |
|---|---|---|---|---|---|
| (NBu4)[MnCr(C6O4Br2)3]·C6H5Br·0.5H2O (A) a | 9.9557(5) | 23.6054(10) | 12.2129(6) | 105.187(5) | 2769.9(2) |
| (NBu4)[MnCr(C6O4Br2)3]·C6H5Br·0.5H2O (A) b | 10.104(4) | 23.70(1) | 12.363(5) | 106.78(4) | 2834.17 |
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5Cl (1) | 9.799(8) | 23.76(3) | 12.08(1) | 104.71(8) | 2719.83 |
| (NBu4)[MnCr(C6O4Cl2)3]·1.5C6H5Br (2) | 9.89(1) | 23.66(4) | 12.06(2) | 103.7(1) | 2740.18 |
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5I (3) | 9.71(4) | 23.6(1) | 12.23(6) | 104.3(5) | 2720.50 |
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5CH3 (4) | 9.822(7) | 23.88(3) | 12.09(1) | 104.95(8) | 2739.65 |
| (NBu4)[MnCr(C6O4Cl2)3]·2C6H5CN (5) | 9.887(9) | 23.55(3) | 12.08(1) | 104.5(1) | 2723.03 |
| (NBu4)[MnCr(C6O4Cl2)3]·2C6H5NO2 (6) | 9.58(4) | 23.7(1) | 11.98(6) | 105.8(4) | 2611.89 |
| Compound | χmT @ 300 K (cm3 K mol−1) | Tc (K) | M @ 5 T (μB) | Hc (mT) |
|---|---|---|---|---|
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5Cl (1) | 6.29 | 10.4 | 2.20 | 43.7 |
| (NBu4)[MnCr(C6O4Cl2)3]·1.5C6H5Br (2) | 6.22 | 10.7 | 2.15 | 44.7 |
| (NBu4)[MnCr(C6O4Cl2)3]·C6H5I (3) | 6.26 | 11.0 | 2.11 | 30.2 |
| (NBu4)[MnCr(C6O4Cl2)3]· C6H5CH3 (4) | 6.26 | 9.8 | 2.20 | 16.2 |
| (NBu4)[MnCr(C6O4Cl2)3]·2C6H5CN (5) | 6.27 | 10.8 | 2.20 | 19.3 |
| (NBu4)[MnCr(C6O4Cl2)3]·2C6H5NO2 (6) | 6.30 | 11.2 | 2.12 | 56.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, C.; Benmansour, S.; Gómez-García, C.J. Chloranilato-Based Layered Ferrimagnets with Solvent-Dependent Ordering Temperatures. Magnetochemistry 2019, 5, 34. https://doi.org/10.3390/magnetochemistry5020034
Martínez-Hernández C, Benmansour S, Gómez-García CJ. Chloranilato-Based Layered Ferrimagnets with Solvent-Dependent Ordering Temperatures. Magnetochemistry. 2019; 5(2):34. https://doi.org/10.3390/magnetochemistry5020034
Chicago/Turabian StyleMartínez-Hernández, Cristian, Samia Benmansour, and Carlos J. Gómez-García. 2019. "Chloranilato-Based Layered Ferrimagnets with Solvent-Dependent Ordering Temperatures" Magnetochemistry 5, no. 2: 34. https://doi.org/10.3390/magnetochemistry5020034
APA StyleMartínez-Hernández, C., Benmansour, S., & Gómez-García, C. J. (2019). Chloranilato-Based Layered Ferrimagnets with Solvent-Dependent Ordering Temperatures. Magnetochemistry, 5(2), 34. https://doi.org/10.3390/magnetochemistry5020034