Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine
Abstract
:1. Introduction
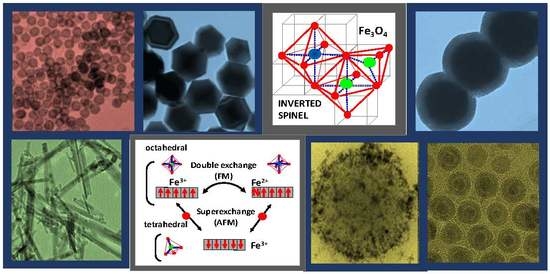
2. Magnetite: Structure and Properties from Bulk to Nanoscale
- The large contribution of surface ions, located on edges or corners, with coordination numbers lower than inner core ions, (see Figure 3) gives rise to an abrupt breakdown of the lattice and magnetic symmetry, which induces changes in magnetic anisotropy at the surface.
- The chemical environment of the coating shell influences the magnetic properties of the surface ions.
- The interaction of the surface spins with the inner ones by an exchange bias may lead to some degree of frustration that lowers the total magnetization of the nanoparticle [28].
3. Synthetic Procedures of Multifunctional SPM Magnetite Nanostructures
3.1. Synthesis of the Magnetic Core. Synthetic Procedures Modulating Size and Shape
3.1.1. Precipitation Methods
3.1.2. Thermal Decomposition
3.1.3. Hydrothermal Synthesis
3.1.4. Solvothermal Procedures
3.1.5. Biogenic Inspired Procedures
3.2. Surface Functionalization: Core Protection, Colloidal Stability, Biocompatibility and Multifunctional Decoration
3.2.1. Small Organic Molecules
3.2.2. Large Polymers
3.2.3. Inorganic Materials
4. Biomedical Applications Arising from the Tunable Magnetic Properties of Magnetite Nanoparticles
4.1. Magnetophoresis
4.1.1. Cell Isolation
4.1.2. Magnetofection
4.1.3. Magnetic Guiding
4.2. Magnetic Detection
4.2.1. Superconducting Quantum Interference Device
4.2.2. Giant Magnetoresistance (GMR)
4.2.3. Impedance Based Sensor
4.3. Inductive Heating
4.3.1. Cancer Hyperthermia Treatment
4.3.2. Controlled Release
4.3.3. Thermal Stimulation
4.4. Magnetic Resonance Imaging
4.5. Magnetic Prototyping of Functional Biological Structures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jacobs, I.S. Role of Magnetism in Technology. J. Appl. Phys. 1969, 40, 917. [Google Scholar] [CrossRef]
- Jacobs, S.; Bean, C.P. Fine Particles, Thin Films and Exchange Anisotropy. In Magnetism; Rado, G.T., Suhl, H., Eds.; Academic: New York, NY, USA, 1963; Volume 3, pp. 271–350. [Google Scholar]
- Guéron, S.; Deshmukh, M.M.; Myers, E.B.; Ralph, D.C. Tunneling via Individual Electronic States in Ferromagnetic Nanoparticles. Phys. Rev. Lett. 1999, 83, 4148–4151. [Google Scholar] [CrossRef] [Green Version]
- Liou, S.H.; Huang, S.; Klimek, E.; Kirby, R.D.; Yao, Y.D. Enhancement of coercivity in nanometer-size CoPt crystallites. J. Appl. Phys. 1999, 85, 4334–4336. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Amiji, M.; Shenoy, D.; Nagesha, D.; Weissig, V.; Fu, W. Nanomedicine: A new paradigm in diagnosis and therapy. Optics East 2005 2005, 6008, 600816. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Vargas, Z.; Rivas, J.; López-Quintela, M.A. Iron Oxide based Nanoparticles for Magnetic Hyperthermia Strategies in Biomedical Applications. Eur. J. Inorg. Chem. 2015, 27, 4495–4509. [Google Scholar] [CrossRef]
- Dobson, J. Nanoscale biogenic iron oxides and neurodegenerative disease. FEBS Lett. 2001, 496, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Cabrer, P.; Campos, F. Liposomes and nanotechnology in drug development: Focus on neurological targets. Int. J. Nanomed. 2013, 8, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400–13402. [Google Scholar]
- NanoTherm. Available online: https://www.magforce.com/en/home/our_therapy/ (accessed on 28 July 2019).
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Ortolani, A.; Bianchi, M.; Mosca, M.; Caravelli, S.; Fuiano, M.; Marcacci, M.; Russo, A. The prospective opportunities offered by magnetic scaffolds for bone tissue engineering: A review. Joints 2016, 4, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-VCH: Wienheim, Germany, 2003. [Google Scholar]
- Banerjee, S.K.; Moskowitz, B.M. Magnetite Biomineralization and Magnetoreception in Organisms, a New Biomagnetism; Kirschvink, J.L., Jones, D.S., MacFadden, B.J., Eds.; Chapter 2: Ferrimagnetic Properties of Magnetite; Springer: New York, NY, USA, 1985. [Google Scholar]
- Parkinson, G.S. Iron Oxide Surfaces. Surface Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef] [Green Version]
- Verwey, E.J.W. Electronic Conduction of Magnetite (Fe3O4) and Its Transition Point at Low Temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Senn, M.S.; Wright, J.P.; Attfield, J.P. Charge Order and Three-Site Distortions in the Verwey Structure of Magnetite. Nature 2012, 481, 173–176. [Google Scholar] [CrossRef]
- Goodenough, J.B. Magnetism and the Chemical Bond; Wiley-Interscience: New York, NY, USA, 1963. [Google Scholar]
- Skomski, R.; Sellmeyer, D. Cap.1, Handbook of Advanced Magnetic Materials; Sellmeyer, D.J., Liu, Y., Shindo, D., Eds.; Tingshua University Press: New York, NY, USA, 2006. [Google Scholar]
- Landau, L.D.; Lifshitz, E. On the theory of the dispersion of magnetic permeability in ferromagnetic bodies. Phys. Z. Sowjetunion 1935, 8, 153–169. [Google Scholar]
- Blundell, S. Magnetism in Condensed Matter; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Krishnan, K.M. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. IEEE Trans. Magn. 2010, 46, 2523. [Google Scholar]
- Yoon, K.Y.; Xue, Z.; Fei, Y.; Lee, J.H.; Cheng, V.; Bagaria, H.G.; Huh, C.; Bryant, S.L.; Kong, S.D.; Ngo, V.W.; et al. Control of magnetite primary particlesize in aqueous dispersions of nanoclusters for high magnetic susceptibilities. J. Colloid Interface Sci. 2015, 462, 359–367. [Google Scholar] [CrossRef]
- Vargas-Osorio, Z.; Argibay, B.; Piñeiro, Y.; Vázquez-Vázquez, C.; López-Quintela, M.A.; Álvarez-Pérez, M.A.; Sobrino, T.; Campos, F.; Castillo, J.; Rivas, J. Multicore Magnetic Fe3O4@C Beads with Enhanced Magnetic Response for MRI in Brain Biomedical Applications. IEEE Trans. Magn. 2016, 52, 230604. [Google Scholar] [CrossRef]
- Cótica, L.F.; Santos, I.A.; Girotto, E.M.; Ferri, E.V.; Coelho, A.A. Surface spin disorder effects in magnetite and poly(thiophene)-coated magnetite nanoparticles. J. Appl. Phys. 2010, 108, 064325. [Google Scholar] [CrossRef] [Green Version]
- Nedelkoski, Z.; Kepaptsoglou, D.; Lari, L.; Wen, T.; Booth, R.A.; Oberdick, S.D.; Galindo, L.; Ramasse, Q.M.; Evans, R.F.L.; Majetich, S.; et al. Origin of reduced magnetization and domain formation in small magnetite nanoparticles. Sci. Rep. 2017, 7, 45997. [Google Scholar] [CrossRef]
- Fiorani, D. Surface Effects in Magnetic Nanoparticles; Fiorani, D., Ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Lacroix, L.-M.; Lachaize, S.; Falqui, A.; Blon, T.; Carrey, J.; Dumestre, F.; Amiens, C.; Margeat, O.; Chaudret, B.; Lecante, P.; et al. Ultrasmall iron nanoparticles: Effect of size reduction on anisotropy and magnetization. J. Appl. Phys. 2008, 103, 7. [Google Scholar] [CrossRef]
- Park, J.; An, K.J.; Hwang, Y.S.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Labarta, A.; Batlle, X. Tuning the Size, the Shape, and the Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2011, 115, 390–396. [Google Scholar] [CrossRef]
- Verma, J.; Lal, S.; van Noorden, C.J.F. Nanoparticles for hyperthermic therapy synthesis strategies and applications in glioblastoma. Int. J. Nanomed. 2014, 9, 2863–2877. [Google Scholar]
- Bañobre-López, M.; Piñeiro Redondo, Y.; López-Quintela, M.A.; Rivas, J. Handbook of Nanomaterials Properties; Bhushan, B., Luo, D., Schricker, S., Sigmund, W., Zauscher, S., Eds.; Chapter 15: Magnetic Nanoparticles for Biomedical Applications; Springer-Verlag: Berlin, Germany, 2014. [Google Scholar]
- La Mer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijevic, E.J. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Peng, S.; Sun, S. Synthesis and Characterization of Monodisperse Hollow Fe3O4 Nanoparticles. Angew. Chem. 2007, 119, 4233–4236. [Google Scholar] [CrossRef]
- Xiaohui, L.; Guangbin, J.; Yousong, L.; Huang, O.; Yang, Z.; Du, Y. Formation mechanism and magnetic properties of hollow Fe3O4 nanospheres synthesized without any surfactant. Cryst. Eng. Comm. 2012, 14, 8658–8663. [Google Scholar]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef] [PubMed]
- Dokyoon, K.; Nohyun, L.; Mihyun, P.B.; Hyo, K.K.; An Taeghwan, H. Synthesis of Uniform Ferrimagnetic Magnetite Nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar]
- Kolen’ko, Y.; Bañobre-López, M.; Rodriguez-Abreu, C.; Carbo-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.; Kovnir, K.; Lebedev, O.; et al. Large-Scale Synthesis of Colloidal Fe3O4 Nanoparticles Exhibiting High Heating Efficiency in Magnetic Hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Begin-Colin, S.; Greneche, J.-M.; Ulhaq-Bouillet, C.; Légaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal Synthesis of Monodisperse Magnetite Nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.B.; Chen, Q.W.; Yu, Y.F.; Cheng, K. Synthesis of carbon-encapsulated superparamagnetic colloidal nanoparticles with magnetic-responsive photonic crystal property. Dalton Trans. 2010, 39, 9565–9569. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.W.; Yu, Y.F.; Cheng, K.; Sun, Y.B. Size- and solvent-dependent magnetically responsive optical diffraction of carbon encapsulated superparamagnetic colloidal photonic crystals. J. Phys. Chem. C 2011, 115, 11427–11434. [Google Scholar] [CrossRef]
- Gorobets, O.; Gorobets, S.; Koralewski, M. Physiological origin of biogenic magnetic nanoparticles in health and disease: From bacteria to humans. Int. J. Nanomed. 2017, 12, 4371–4395. [Google Scholar] [CrossRef] [Green Version]
- Uchida, L.; Liepold, O.; Douglas, T. Protein Cage Magnetic Nanoparticles in Magnetic Nanoparticles from Fabrication to Clinical Applications; Thanh, N.T.K., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2012; pp. 73–95. [Google Scholar]
- Meldrun, F.C.; Wade, V.J.; Nimmo, D.L.; Heywood, B.R.; Mann, S. Synthesis of inorganic nanophase materials in supramolecular protein cages. Nature 1991, 349, 684–687. [Google Scholar] [CrossRef]
- Fantechi, E.; Innocenti, C.; Zanardelli, M.; Fittipaldi, M.; Falvo, E.; Carbo, M.; Shullani, V.; Manelli, C.; Ghelardini, C.; Ferret, A.M.; et al. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 2014, 8, 4705–4719. [Google Scholar] [CrossRef]
- Parker, M.J.; Allen, M.A.; Ramsay, B.; Klem, M.T.; Young, M.; Douglas, T. Expanding the temperature range of biomimetic synthesis using a ferritin from the hyperthermophile pyrococcus furiosus. Chem. Mat. 2008, 20, 1541–1547. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nanotoday 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Barui, A.K.; Oh, J.Y.; Jana, B.; Kim, C.; Ryu, J.-H. Cancer-Targeted Nanomedicine: Overcoming the Barrier of the Protein Corona. Adv. Therap. 2019. [Google Scholar] [CrossRef]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, Functionalization, and Design of Magnetic Nanoparticles for Theranostic Applications. Adv. Health Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Quanguo, H.; Lei, Z.; Wei, W.; Rong, H.; Jingke, H. Preparation and Magnetic comparison of Silane-Functionalized Magnetite Nanoparticles. Sens. Mater. 2010, 22, 285–295. [Google Scholar]
- Mashhadizadeh, M.H.; Amoli-Diva, M. Drug-Carrying Amino Silane Coated Magnetic Nanoparticles as Potential Vehicles for Delivery of Antibiotics. J. Nanomed. Nanotechnol. 2012, 3, 1000139. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davies, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, E.; Sukenik, C.N.; Markovich, G. Alkyl Phosphonate/Phosphate Coating on Magnetite Nanoparticles: A Comparison with Fatty Acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Guardia, P.; Batlle-Brugal, B.; Roca, A.G.; Iglesias, O.; Morales, M.P.; Serna, C.J.; Labarta, A.; Batlle, X. Surfactant effects in monodisperse magnetite nanoparticles of controlled size. J. Magn. Magn. Mater. 2007, 316, e756. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef]
- Fan, J.; Lu, J.; Xu, R.; Jiang, R.; Gao, Y. Use of water-dispersible Fe2O3 nanoparticles with narrow size distributions in isolating avidin. J. Colloid Interface Sci. 2003, 266, 215–218. [Google Scholar] [CrossRef]
- Piñeiro-Redondo, Y.; Bañobre-López, M.; Pardiñas-Blanco, I.; Goya, G.; López-Quintela, M.A.; Rivas, J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res. Lett. 2011, 6, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Niu, M.; Qiao, R.; Gao, M. Superdispersible PVP-Coated Fe3O4 Nanocrystals Prepared by a “One-Pot” Reaction. J. Phys. Chem. B 2008, 112, 14390–14394. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Farkas, K.; Földesi, I.; Szekeres, M.; Illés, E.; Tóth, I.Y.; Nesztor, D.; Szabó, T. Polyelectrolyte coating on superparamagnetic iron oxide nanoparticles as interface between magnetic core and biorelevant media. Interface Focus 2016, 6, 20160068. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yan, H. A green and facile approach for synthesis of magnetite nanoparticles with tunable sizes and morphologies. Mater. Lett. 2012, 73, 129–132. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mat. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Gref, R.; Luck, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Muller, R.H. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Rahman, M.; Laurent, S.; Tawil, N.; Yahia, L.H.; Mahmoudi, M. Protein-NP Interactions, the Bio-Nano Interface; Springer Series in Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; Volume 15, pp. 21–44. [Google Scholar]
- Pearson, R.M.; Juettner, V.V.; Hong, S. Biomolecular corona on nanoparticles: A survey of recent literature and its implications in targeted drug delivery. Front. Chem. 2014, 2, 108. [Google Scholar] [CrossRef]
- You, J.O.; Almeda, D.; Ye, G.J.C.; Auguste, D.T. Bioresponsive matrices in drug delivery. J. Biol. Eng. 2010, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yadavalli, T.; Ramasamy, S.; Chandrasekaran, G.; Michael, I.; Therese, H.A.; Chennakesavulu, R. Dual responsive PNIPAM–chitosan targeted magnetic nanopolymers for targeted drug delivery. J. Magn. Magn. Mater. 2015, 380, 315–320. [Google Scholar] [CrossRef]
- Dionigi, C.; Piñeiro, Y.; Riminucci, A.; Bañobre, M.; Rivas, J.; Dediu, V. Regulating the thermal response of PNIPAM hydrogels by controlling the adsorption of magnetite nanoparticles. Appl. Phys. A Mater. Sci. Process 2014, 114, 585–590. [Google Scholar] [CrossRef]
- Digigow, R.G.; Dechéllez, J.F.; Dietsch, H.; Geissbühler, I.; Vanhecke, D.; Geers, C.; Hirt, A.M.; Rothen-Rutishauer, B. Preparation and characterization of functional silicahybrid magnetic nanoparticles. J. Magn. Magn. Mater. 2014, 362, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Osorio, Z.; González-Gómez, M.A.; Piñeiro, Y.; Vázquez-Vázquez, C.; Rodríguez-Abreu, C.; López-Quintela, M.A.; Rivas, J. Novel synthetic routes of large-pore magnetic mesoporous nanocomposites (SBA-15/Fe3O4) as potential multifunctional theranostic nanodevices. J. Mater. Chem. B 2017, 5, 9395–9404. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.; Ko, S.; Kim, I.; Huang, Y.; Mohanty, K.K.; Huh, C.; Maynard, J.A. Recent Advances Incorporating Superparamagnetic Nanoparticles into Immunoassays. ACS Appl. Nano Mater. 2018, 1, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Hibino, E.; Shimizu, K.; Kobayashi, T.; Yamada, Y.; Hibi, H.; Ueda, M.; Honda, H.J. Magnetic force—Based mesenchymal stem cell expansion using antibody—Conjugated magnetoliposomes. Biomed. Mater. Res. Part B 2005, 75B, 320–327. [Google Scholar] [CrossRef]
- Chao, C.; Jun, W.; Yong, Z.; Cong, L.; Lina, X.; Xin, Z.; Xiaolan, Y.; Ming, L.; Yang, B.; Tingyu, L.; et al. Improving Magnetofection of Magnetic Polyethylenimine Nanoparticles into MG-63 Osteoblasts using a Novel Uniform Magnetic Field. Nanoscale Res. Lett. 2019, 14, 90. [Google Scholar]
- Chorny, M.; Fishbein, I.; Yellen, B.B.; Alferiev, I.S.; Bakay, M.; Ganta, S.; Adamo, R.; Amiji, M.; Friedman, G.; Levy, R.J. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl. Acad. Sci. USA 2010, 107, 8346–8351. [Google Scholar] [CrossRef] [Green Version]
- Ryhänen, T.; Seppä, H.; Ilmoniemi, R.; Knuutila, J. SQUID magnetometers for low-frequency applications. J. Low Temp. Phys. 1989, 76, 287–386. [Google Scholar] [CrossRef]
- Pankhurst, Q.; Hautot, D.; Khan, N.; Dobson, J. Increased Levels of Magnetic Iron Compounds in Alzheimer’s Disease. J. Alzheimer’s Dis. 2008, 13, 49–52. [Google Scholar] [CrossRef]
- Tanaka, S.; Ota, H.; Kondo, Y.; Tamaki, Y.; Kobayashi, S.; Noguchi, S. Detection of magnetic nanoparticles in lymph nodes of rat by high Tc SQUID. IEEE Trans. Appl. Supercond. 2003, 13, 377–380. [Google Scholar] [CrossRef]
- Adolphi, N.L.; Butler, K.S.; Lovato, D.M.; Tessier, T.E.; Trujillo, J.E.; Hathaway, H.J.; Fegan, D.L.; Monson, T.C.; Stevens, T.E.; Huber, D.L.; et al. Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: Comparison of SQUID-detected magnetic relaxometry and MRI. Contrast Media Mol. Imaging 2012, 7, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Bhuiya, A.K.; Asai, M.; Watanabe, H.; Hirata, T.; Higuchi, Y.; Yoshida, T.; Enpuku, K. Characterization of magnetic markers and sensors for liquid-phase immunoassays using Brownian relaxation. IEEE Trans. Magn. 2012, 48, 2838–2841. [Google Scholar] [CrossRef]
- Cubells-Beltrán, M.D.; Reig, C.; Madrenas, J.; De Marcellis, A.; Santos, J.; Cardoso, S.; Freitas, P.P. Integration of GMR Sensors with Different Technologies. Sensors 2016, 16, 939. [Google Scholar] [CrossRef] [PubMed]
- Osterfeld, S.J.; Yu, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.-J.; Hall, E.A.; Wilson, R.J.; Sun, S.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 2008, 105, 20637–20640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Tu, L.; Klein, T.; Feng, Y.; Wang, J.P. Surface Modification for Protein and DNA Immobilization onto GMR Biosensor. IEEE Trans. Magn. 2013, 49, 296–299. [Google Scholar] [CrossRef]
- Lago-Cachón, D.; Rivas, M.; Martínez-García, J.C.; García, J.A. Cu impedance-based detection of superparamagnetic nanoparticles. Nanotechnology 2013, 24, 245501. [Google Scholar] [CrossRef]
- Lago-Cachon, D.; Oliveira-Rodriguez, M.; Moyano, A.; Rivas, M.; Blanco-Lopez, M.C.; Martinez-Garcia, J.C.; Salvador, M.; Garcia, J.A. Scanning Magneto-Inductive Sensor for Quantitative Assay of Prostate-Specific Antigen. IEEE Magn. Lett. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Moyano, A.; Salvador, M.; Martínez, J.C.; Rivas, M.; Blanco-López, M.C.; Gónzalez-Gómez, M.A.; Yáñez, S.; Piñeiro, Y.; Rivas, J. Carbon-coated superparamagnetic nanoflowers as labels in lateral flow imunoassays. In Proceedings of the 10th International Conference on Fine Particle Magnetism, Gijon, Spain, 27–30 May 2019; Book of Abstracts. p. 187. [Google Scholar]
- Piñeiro-Redondo, Y.; Vargas-Osorio, Z.; Bañobre-López, M.; Kolen’ko, Y.V.; López-Quintela, M.A.; Rivas, J. Relevant Parameters for Magnetic Hyperthermia in Biological: Applications: Agglomeration, Concentration, and Viscosity. IEEE Trans. Magn. 2016, 52, 2300704(1-4). [Google Scholar]
- Hergt, R.; Andrä, W. Magnetism in Medicine: A Handbook, 2nd ed.; Andrä, W., Nowak, H., Eds.; Chapter 4: Magnetic Hyperthermia and Thermoablation; Wiley-VCH: Berlin, Germany, 2007. [Google Scholar]
- Minelli, C.; Lowe, S.B.; Stevens, M.S. Engineering Nanocomposite Materials for Cancer therapy. Small 2010, 6, 2336–2357. [Google Scholar] [CrossRef]
- MagForce AG. Available online: https://www.magforce.com/en/home/our_therapy/ (accessed on 29 September 2019).
- Dionigi, C.; Lungaro, L.; Goranov, V.; Riminucci, A.; Piñeiro-Redondo, Y.; Bañobre-López, M.; Rivas, J.; Dediu, V. Smart magnetic poly(N-isopropylacrylamide) to control the release of bio-active molecules. J. Mater. Sci. Mater. Electron. 2014, 25, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Meikle, S.; Pineiro, Y.; Bañobre Lopez, M.; Rivas, J.; Santin, M. Mild hyperthermia nano-devices based on superparamagnetic nanoparticles conjugated with thermoresponsive poly(epsilon-lysine) dendrons tethered with carboxybetaine. Acta Biomater. 2016, 40, 235–242. [Google Scholar]
- Chen, R.; Romero, G.; Christiansen, M.G.; Mohr, A.; Anikeeva, P. Wireless magnetothermal deep brain stimulation, Sciencexpress. Science 2015, 347, 1477–1480. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; Guduru, R.; Hong, J.; Llang, P.; Nair, M.; Khizroev, S. Magneto-electric Nanoparticules for Non-Invasive Brain Stimulation. PLoS ONE 2012, 7, e44040. [Google Scholar] [CrossRef] [Green Version]
- Carril, M.; Fernández, I.; Rodriguez, J.; García, I.; Peñadés, S. Gold-coated iron oxide glyconanoparticles for MRI, CT, and US multimodal imaging. Part. Part. Syst. Charact. 2014, 31, 81–87. [Google Scholar] [CrossRef]
- Jang, J.-T.; Nah, H.; Lee, J.-H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant-Controlled Magnetic Nanoparticles. Angew. Chem. 2009, 121, 1260–1264. [Google Scholar] [CrossRef]
- Gupte, A.A.; Shrivastava, D.; Spaniol, M.A.; Abosch, A. MRI-related heating near deep brain stimulation electrodes: More data are needed. Ster. Funct. Neurosurg. 2011, 89, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T.; Lee, J. Water-Dispersible Ferrimagnetic Iron Oxide Nanocubes with Extremely High r2 Relaxivity for Highly Sensitive in Vivo MRI of Tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting Enhanced Permeability and Retention Effect Driven Delivery by Ultrafine Iron Oxide Nanoparticles with T1–T2 Switchable Magnetic Resonance Imaging Contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef] [Green Version]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomater 2018, 180, 52–66. [Google Scholar] [CrossRef] [PubMed]

















| Material | Fe | Co | Ni | PtFe | Fe3 O4 | γ-Fe2O3 |
| Kan (kJ/m3) | 48 | 530 | 4.8 | 6600 | 11 | 4.6 |
| TC (K) | 1043 | 1388 | 631 | 750 | 858 | 863 |
| Magnetic Property | Application | Pros/Cons. | Commercial Kit | Toxicity |
|---|---|---|---|---|
| Magnetophoresis | Cell isolation |
| MACS® Separators, Miltenyi | No in vitro toxicity |
| Magnetofection |
| NONE | No in vitro toxicity | |
| Magnetic guiding |
| NONE | no in vivo toxicity tested in rat | |
| Magnetic detection | Magnetometry SQUID |
| NONE | No in vitro toxicity |
| GMR sensors |
| NONE | No in vitro toxicity | |
| Impedance Sensors |
| NONE | No in vitro toxicity | |
| Inductive heating | Hyperthermia cancer treatment |
| MAGFORCE NanoTherm, (clinical therapy) | NONE |
| Thermally Enhanced release of therapeutic agents |
| NONE | No in vitro toxicity | |
| Thermal stimulation |
| NONE | no in vivo toxicity tested in mouse | |
| Magneticre laxation | MRI contrast agents: single (T2) |
| Ferucarbotran (Resovist®, Bayer Healthcare) (MRI clinical diagnosis) | NONE |
| MRI contrast agents: dual(T1/T2) |
| NONE | no in vivo toxicity tested in mouse | |
| Magnetic prototyping | 3D magnetic bioprinting |
| NONE | No in vitro and ex vivo toxicity |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñeiro, Y.; González Gómez, M.; de Castro Alves, L.; Arnosa Prieto, A.; García Acevedo, P.; Seco Gudiña, R.; Puig, J.; Teijeiro, C.; Yáñez Vilar, S.; Rivas, J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry 2020, 6, 4. https://doi.org/10.3390/magnetochemistry6010004
Piñeiro Y, González Gómez M, de Castro Alves L, Arnosa Prieto A, García Acevedo P, Seco Gudiña R, Puig J, Teijeiro C, Yáñez Vilar S, Rivas J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry. 2020; 6(1):4. https://doi.org/10.3390/magnetochemistry6010004
Chicago/Turabian StylePiñeiro, Yolanda, Manuel González Gómez, Lisandra de Castro Alves, Angela Arnosa Prieto, Pelayo García Acevedo, Román Seco Gudiña, Julieta Puig, Carmen Teijeiro, Susana Yáñez Vilar, and José Rivas. 2020. "Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine" Magnetochemistry 6, no. 1: 4. https://doi.org/10.3390/magnetochemistry6010004
APA StylePiñeiro, Y., González Gómez, M., de Castro Alves, L., Arnosa Prieto, A., García Acevedo, P., Seco Gudiña, R., Puig, J., Teijeiro, C., Yáñez Vilar, S., & Rivas, J. (2020). Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry, 6(1), 4. https://doi.org/10.3390/magnetochemistry6010004