When Molecular Magnetism Meets Supramolecular Chemistry: Multifunctional and Multiresponsive Dicopper(II) Metallacyclophanes as Proof-of-Concept for Single-Molecule Spintronics and Quantum Computing Technologies?
Abstract
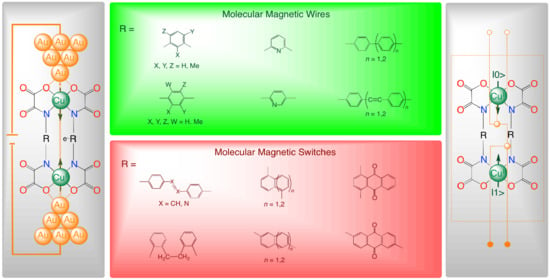
:1. Introduction and Background: Molecular Magnetism Meets Metallosupramolecular Chemistry for Single-Molecule Spintronics and Quantum Computing
2. Dinuclear Copper(II) Metallacyclophanes in the Proof-of-Concept (POC) Design of Molecular Magnetic Wires
2.1. Non-Polyradical Spacers
2.2. Polyradical Spacers
3. Dinuclear Copper(II) Metallacyclophanes in the POC Design of Molecular Magnetic Switches
3.1. Chemoactive Spacers
3.2. Electroactive Spacers
3.3. Photoactive Spacers
4. Conclusions and Outlook: Metallosupramolecular Chemistry Acts as a Rail from Single-Molecule Spintronics to Quantum Computing
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Constable, E.C. Higher oligopyridines as a structural motif in metallosupramolecular chemistry. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: New York, NY, USA, 1994; Volume 42, pp. 67–138. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Sauvage, J.P. (Ed.) Transition Metals in Supramolecular Chemistry; Wiley: New York, NY, USA, 1999; Volume 5. [Google Scholar]
- Swiegers, G.F.; Malefetse, T.J. New self-assembled structural motifs in coordination chemistry. Chem. Rev. 2000, 100, 3483–3538. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and fucntionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Lescop, C. Coordination-driven synthesis of compact supramolecular metallacycles towards extended metallo-organic stacked supramolecular assemblies. Acc. Chem. Res. 2017, 50, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Pilgrim, B.S.; Nitschke, J.R. Covalent post-assembly modification in metallosupramolecular chemistry. Chem. Soc. Rev. 2018, 47, 626–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobylarczyk, J.; Kuzniak, E.; Liberka, M.; Chorazy, S.; Sieklucka, B.; Podjajny, R. Modular approach towards functional multimetallic coordination clusters. Coord. Chem. Rev. 2020, 419, 213394. [Google Scholar] [CrossRef]
- Magnetism: A Supramolecular Function; NATO ASI Series C; Kahn, O. (Ed.) NATO ASI Series C; Kluwer: Dordrecht, The Netherlands, 1996; Volume 484. [Google Scholar]
- Thompson, L.K. Magnetism-molecular and supramolecular perspectives. Coord. Chem. Rev. 2005, 249, 2549. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Cano, J.; Ottenwaelder, X.; Lescouëzec, R.; Journaux, Y.; Lloret, F.; Julve, M. Ligand design for multidimensional magnetic materials: A metallosupramolecular perspective. Dalton Trans. 2008, 2780–2805. [Google Scholar] [CrossRef]
- Kepert, C.J. Supramolecular magnetic materials. Aust. J. Chem. 2009, 62, 1079–1080. [Google Scholar] [CrossRef]
- Weiss, J. Supramolecular approaches to nano and molecular electronics. Coord. Chem. Rev. 2010, 254, 2247–2248. [Google Scholar] [CrossRef]
- Dul, M.C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular coordination chemistry of aromatic polyoxalamide ligands: A metallosupramolecular approach toward functional magnetic materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Sanvito, S. Injecting and controlling spins in organic materials. J. Mater. Chem. 2007, 17, 4455–4459. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef]
- Metzger, R.M. Unimolecular electronics. Chem. Rev. 2015, 115, 5056–5115. [Google Scholar] [CrossRef]
- Bogani, L. Experiments on molecular nanomagnets for molecular spintronics. Struct. Bond. 2015, 164, 331–381. [Google Scholar]
- Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X. Molecular-scale electronics: From concept to function. Chem. Rev. 2016, 116, 4318–4440. [Google Scholar] [CrossRef]
- Cinchetti, M.; Dediu, V.A.; Hueso, L.E. Activating the molecular spinterface. Nat. Mater. 2017, 16, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Novak, M.A.; Del Barco, E. Molecular spintronics. J. Appl. Phys. 2019, 125, 240401. [Google Scholar] [CrossRef]
- Guo, L.; Gu, X.; Zhu, X.; Sun, X. Recent advances in molecular spintronics: Multifunctional spintronic devices. Adv. Mater. 2019, 31, 1805355. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-S.; Tang, Z.; Tao, J. Bistable molecular materials with dynamic structures. Chem. Commun. 2020, 56, 2071–2086. [Google Scholar] [CrossRef]
- Winpenny, R.E.P. Quantum information processing using molecular nanomagnets as qubits. Angew. Chem. Int. Ed. 2008, 47, 7992–7994. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaita-Ariño, A.; Coronado, E.; Loss, D. Quantum computing with molecular spin systems. J. Mater. Chem. 2009, 19, 1672–1677. [Google Scholar] [CrossRef]
- Stamp, P.C.E.; Gaita-Ariño, A. Spin-based quantum computers made by chemistry: Hows and whys. J. Mater. Chem. 2009, 19, 1718–1730. [Google Scholar] [CrossRef] [Green Version]
- Ardavan, A.; Blundell, S.J. Storing quantum information in chemically engineered nanoscale magnets. J. Mater. Chem. 2009, 19, 1754–1760. [Google Scholar] [CrossRef]
- Dei, A.; Gatteschi, D. Molecular (nano)magnets as test grounds of quantum mechanics. Angew. Chem. Int. Ed. 2011, 50, 11852–11858. [Google Scholar] [CrossRef]
- Timco, G.A.; Faust, T.B.; Tuna, F.; Winpenny, R.E.P. Linking heterometallic rings for quantum information processing and amusement. Chem. Soc. Rev. 2011, 40, 3067–3075. [Google Scholar] [CrossRef]
- Troiani, F.; Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev. 2011, 40, 3119–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aromí, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Daly, B.; Silverson, V.A.D.; De Silva, A.P. Taking baby steps in molecular logic-based computation. Chem. Commun. 2015, 51, 8403–8409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghirri, A.; Troiani, F.; Affronte, M. Quantum computation with molecular nanomagnets: Achievements, challenges, and new trends. Struct. Bond. 2015, 164, 383–430. [Google Scholar]
- Sessoli, R. Toward the quantum computer: Magnetic molecules back in the race. ACS Cent. Sci. 2015, 1, 473–474. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.-S.; Deng, Y.-F.; Zheng, Y.-Z. The rise of single-ion magnets as spin qubits. Magnetochemistry 2016, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Palii, A.; Tsukerblat, B. Tuning of quantum entanglement in molecular quantum cellular automata based on mixed-valence tetrameric units. Dalton Trans. 2016, 45, 16661–16672. [Google Scholar] [CrossRef]
- Palii, A.; Tsukerblat, B.; Clemente-Juan, J.-M.; Coronado, E. Spin switching in molecular quantum cellular automata based on mixed-valence tetrameric units. J. Phys. Chem. C 2016, 120, 16694–17005. [Google Scholar] [CrossRef]
- Moreno-Pineda, E.; Godfrin, C.; Balestro, F.; Wernsdorfer, W.; Ruben, M. Molecular spin qudits for quantum algorithms. Chem. Soc. Rev. 2018, 47, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Atzori, M.; Sessoli, R. The second quantum revolution: Role and challenges of molecular chemistry. J. Am. Chem. Soc. 2019, 141, 11339–11352. [Google Scholar] [CrossRef]
- Wasielewski, M.R.; Forbes, M.D.E.; Frank, N.L.; Kowalski, K.; Scholes, G.D.; Yuen-Zhou, J.; Baldo, M.A.; Freedman, D.E.; Goldsmith, R.H.; Goodson III, T.; et al. Exploiting chemistry and molecular systems for quantum information science. Nat. Rev. Chem. 2020, 4, 490–504. [Google Scholar] [CrossRef]
- Liu, I.-P.-C.; Wang, W.-Z.; Peng, S.-M. New generation of metal-string complexes: Strengthening metal-metal interactions via naphthyridyl group modulated oligo-α-pyridylamido ligands. Chem. Commun. 2009, 4323–4331. [Google Scholar] [CrossRef]
- Berry, J.F. Metal-metal bonds in chains of three or more metal atoms: From homometallic to heterometallic chains. Struct. Bond. 2010, 136, 1–28. [Google Scholar]
- Aromí, G. Metal-based molecular chains: Design by coordination chemistry. Comment Inorg. Chem. 2011, 32, 163–194. [Google Scholar] [CrossRef]
- Dul, M.-C.; Pardo, E.; Lezcouëzec, R.; Chamoreau, L.-M.; Villain, F.; Journaux, Y.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; et al. Redox switch-off of the ferromagnetic coupling in a mixed-spin tricobalt(II) triple mesocate. J. Am. Chem. Soc. 2009, 131, 14614–14615. [Google Scholar] [CrossRef]
- Morita, Y.; Yakiyama, Y.; Nakazawa, S.; Murata, T.; Ise, T.; Hashizuma, D.; Shiomi, D.; Sato, K.; Kitagawa, M.; Nakasuji, K.; et al. Triple-stranded metallo-hellicates addressable as Lloyd’s electron spin qubits. J. Am. Chem. Soc. 2010, 132, 6944–6946. [Google Scholar] [CrossRef]
- Luis, F.; Repollés, A.; Martínez-Pérez, M.J.; Aguilà, D.; Roubeau, O.; Zueco, D.; Alonso, P.J.; Evangelisti, M.; Camón, A.; Sesé, J.; et al. Molecular prototypes for spin-based CNOT and SWAP quantum gates. Phys. Rev. Lett. 2011, 107, 117203. [Google Scholar] [CrossRef] [Green Version]
- Aguilà, D.; Barrios, L.E.; Velasco, V.; Roubeau, O.; Repollés, A.; Alonso, P.J.; Sesé, J.; Teat, S.J.; Luis, F.; Aromí, G. Heterodimetallic [LnLn’] lanthanide complexes: Toward a chemical design of two-qubit molecular spin quantum gates. J. Am. Chem. Soc. 2014, 136, 14215–14222. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Soria, J.; Fabelo, O.; Castellano, M.; Cano, J.; Fordham, S.; Zhou, H.-C. Multielectron oxidation in a ferromagnetically coupled dinickel(II) triple mesocate. Chem. Commun. 2015, 51, 13381–13383. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, S.; Feltham, H.L.C.; Brooker, S. Non-porous iron(II)-based sensor: Crystallographic insights into a cycle of colorful guest-induced topotactic transformations. Angew. Chem. Int. Ed. 2016, 55, 15067–15071. [Google Scholar] [CrossRef]
- Darawsheh, M.; Barrios, L.A.; Roubeau, O.; Teat, S.J.; Aromí, G. Guest-, light- and thermally-modulated spin crossover in [FeII2] supramolecular helicates. Chem. Eur. J. 2016, 22, 8635–8645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, O. Magnetic molecular wheels and grids-the need for novel concepts in “zero-dimensional” magnetism. Coord. Chem. Rev. 2005, 249, 2550–2566. [Google Scholar] [CrossRef] [Green Version]
- Timco, G.A.; McInnes, E.J.L.; Winpenny, R.E.P. Physical studies of heterometallic rings: An ideal system for studying magnetically-coupled systems. Chem. Soc. Rev. 2013, 42, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- McInnes, E.J.L.; Timco, G.A.; Whitehead, G.F.S.; Winpenny, R.E.P. Heterometallic rings: Their physics and use as supramolecular building blocks. Angew. Chem. Int. Ed. 2015, 54, 14244–14269. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J. Cr7Ni wheels: Supramolecular tectons for the physical implementation of quantum information processing. Magnetochemistry 2016, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Do Pim, W.D.; De Faria, E.N.; Oliveira, W.X.C.; Pinheiro, C.B.; Nunes, W.C.; Cano, J.; Lloret, F.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. A heterobimetallic [MnII5CuII5] nanowheel modulated by a flexible bis-oxamate type ligand. Dalton Trans. 2015, 44, 10939–10942. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Harris, T.D. An S = 12 semiquinoid radical-bridged Mn6 wheel complex assembled from an asymmetric redox-active bridging ligand. Chem. Commun. 2016, 52, 1006–1008. [Google Scholar] [CrossRef]
- Thompson, L.K. Polynuclear coordination complexes-from dinuclear to nonanuclear and beyond. Coord. Chem. Rev. 2002, 233–234, 193–206. [Google Scholar] [CrossRef]
- Lehn, J.M. Grid-type metal ion architectures: Functional metallosupramolecular arrays. Angew. Chem. Int. Ed. 2004, 43, 3644–3662. [Google Scholar]
- Thompson, L.K.; Waldmann, O.; Xu, Z. Polynuclear manganese grids and clusters—A magnetic perspective. Coord. Chem. Rev. 2005, 249, 2677–2690. [Google Scholar] [CrossRef]
- Ruben, M.; Lehn, J.M.; Müller, P. Addressing metal centres in supramolecular assemblies. Chem. Soc. Rev. 2006, 35, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Dawe, L.N.; Abedin, T.S.M.; Thompson, L.K. Ligand directed self-assembly of polymetallic [n×n] grids: Rational routes to large functional molecular subunits? Dalton Trans. 2008, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G. Metallosupramolecular grid complexes: Towards nanostructured materials with high-tech applications. Chem. Soc. Rev. 2013, 42, 7881–7899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, B.; Demeshko, S.; Dechert, S.; Meyer, F. A double-switching multistable Fe4 grid complex with stepwise spin-crossover and redox transitions. Angew. Chem. Int. Ed. 2010, 49, 9274–9277. [Google Scholar] [CrossRef]
- Schneider, B.; Demeshko, S.; Neudeck, S.; Dechert, S.; Meyer, F. Mixed-spin [2 × 2] Fe4 grid complex optimized for quantum cellular automata. Inorg. Chem. 2013, 52, 13230–13237. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Wang, Y.-T.; Cui, A.-L.; Kou, H.-Z. Toward higher nuclearity: Tetranuclear cobalt(II) metallogrid exhibiting spin crossover. Inorg. Chem. 2014, 53, 2613–2618. [Google Scholar] [CrossRef]
- Matsumoto, T.; Newton, G.N.; Shiga, T.; Hayami, S.; Matsui, Y.; Okamoto, H.; Kumai, R.; Murakami, Y.; Oshio, H. Programmable spin-state switching in a mixed-valence spin crossover iron grid. Nat. Commun. 2016, 5, 3865. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, B.; Greisch, J.F.; Faus, I.; Bodenstein, T.; Salitros, I.; Fuhr, O.; Fink, K.; Schünemann, V.; Kappes, M.M.; Ruben, M. Divergent coordination chemistry: Parallel synthesis of [2 × 2] iron(II) grid-complex tauto-conformers. Angew. Chem. Int. Ed. 2016, 55, 10881–10885. [Google Scholar] [CrossRef]
- Shen, F.; Huang, W.; Wu, D.; Zheng, Z.; Huang, X.-C.; Sato, O. Redox modulation of spin crossover within a cobalt metallogrid. Inorg. Chem. 2016, 55, 902–908. [Google Scholar] [CrossRef]
- Dhers, S.; Mondal, A.; Aguilà, D.; Ramírez, J.; Vela, S.; Dechambenoit, P.; Rouzières, M.; Nitschke, J.R.; Clérac, R.; Lehn, J.M. Spin state chemistry: Modulation of ligand pKa by spin state switching in a [2 × 2] iron(II) grid-type complex. J. Am. Chem. Soc. 2018, 140, 8218–8227. [Google Scholar] [CrossRef] [Green Version]
- Bellini, V.; Lorusso, G.; Candini, A.; Wernsdorfer, W.; Faust, T.B.; Timco, G.A.; Winpenny, R.E.P.; Affronte, M. Propagation of spin information at the supramolecular scale through heteroaromatic linkers. Phys. Rev. Lett. 2011, 106, 227205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiesa, A.; Whitehead, G.F.S.; Carretta, S.; Carthy, L.; Timco, G.A.; Teat, S.J.; Amoretti, G.; Pavarini, E.; Winpenny, R.E.P.; Santini, P. Molecular nanomagnets with switchable coupling for quantum simulation. Sci. Rep. 2014, 4, 07423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrando-Soria, J.; Moreno Pineda, E.; Chiesa, A.; Fernandez, A.; Magee, S.A.; Carretta, S.; Santini, P.; Vitorica-Yrezabal, I.J.; Tuna, F.; Timco, G.A.; et al. A modular design of molecular qubits to implement universal quantum gates. Nat. Commun. 2016, 7, 11377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrando-Soria, J.; Magee, S.A.; Chiesa, A.; Carretta, S.; Santini, P.; Vitorica-Yrezabal, I.J.; Tuna, F.; Whitehead, G.F.S.; Sproules, S.; Lancaster, K.M.; et al. Switchable interaction in molecular double qubits. Chem 2016, 1, 727–752. [Google Scholar] [CrossRef] [Green Version]
- Garlattti, E.; Guidi, T.; Ansbro, S.; Santini, P.; Amoretti, G.; Ollivier, J.; Mutka, H.; Timco, G.; Vitorica-Yrezabal, I.J.; Whitehead, G.F.S.; et al. Portraying entanglement between molecular qubits with four-dimensional inelastic neutron scattering. Nat. Commun. 2017, 8, 14543. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Marvaud, V.; Verdaguer, M.; Marrot, J.; Kalisz, M.; Mathonière, C. Reversible photoinduced magnetic properties in the heptanuclear complex [MoIV(CN)2(CN-CuL)6]8+: A photomagnetic high-spin molecule. Angew. Chem. Int. Ed. 2004, 43, 5468–5471. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Clérac, R.; Roubeau, O.; Harté, E.; Mathonière, C.; Le Bris, R.; Holmes, S.M. Magnetic and optical bistability driven by thermally and photoinduced intramolecular electron transfer in a molecular cobalt-iron Prussian blue analogue. J. Am. Chem. Soc. 2008, 130, 252–258. [Google Scholar] [CrossRef]
- Nihei, M.; Okamoto, Y.; Sekine, Y.; Hoshino, N.; Shiga, T.; Liu, I.-P.-C.; Oshio, H. A light-induced phase exhibingt slow magnetic relaxation in a cyanide-bridged [Fe4Co2] complex. Angew. Chem. Int. Ed. 2012, 51, 6361–6364. [Google Scholar] [CrossRef]
- Garnier, D.; Jiménez, J.-R.; Li, Y.; Von Bardeleben, J.; Journaux, Y.; Augenstein, T.; Moos, E.M.B.; Gamer, M.T.; Breher, F.; Lescouëzec, R. K⊂{[FeII(Tp)(CN)3]4[CoIII(pzTp)]3[CoII(pzTp)]}: A neutral soluble model complex of photomagnetic Prussian blue analogues. Chem. Sci. 2016, 7, 4825–4831. [Google Scholar] [CrossRef] [Green Version]
- Freedman, D.E.; Jenkins, D.M.; Lavarone, A.T.; Long, J.R. A redox-switchable single-molecule magnet incorporating [Re)(CN)7]3−. J. Am. Chem. Soc. 2008, 130, 2884–2885. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Soytherland, H.I.; Avendaño, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clérac, R.; Nehrkorn, J.; et al. Cyanide single-molecule magnets exhibiting solvent dependent reversible “on” and “off” exchange bias behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef] [PubMed]
- Wernsdorfer, W.; Aliaga-Alcalde, N.; Hendrickson, D.N.; Christou, G. Exchange-biased quantum tunneling in a supramolecular dimer of single-molecule magnets. Nature 2002, 416, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Edwards, R.S.; Aliaga-Alcalde, N.; Christou, G. Quantum coherence in an exchange-coupled dimer of single-molecule magnets. Science 2003, 302, 1015–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, F.; Langhirt, M.; Del Barco, E.; Taguchi, T.; Christou, G. Magnetic field dependent transport through a Mn4 single-molecule magnet. J. Appl. Phys. 2011, 109, 07B112. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. A supramolecular aggregate of four exchange-biased single-molecule magnets. J. Am. Chem. Soc. 2011, 133, 20688–20691. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Shiddiq, M.; Ghosh, T.; Abboud, K.A.; Hill, S.; Christou, G. Covalently linked dimer of Mn3 single-molecule magnets and retention of its structure and quantum properties in solution. J. Am. Chem. Soc. 2015, 137, 7160–7168. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Wernsdorfer, W.; Shiddiq, M.; Abboud, K.A.; Hill, S.; Christou, G. Supramolecular aggregates of single-molecule magnets: Exchange-biased quantum tunneling of magnetization in a rectangular [Mn3]4 tetramer. Chem. Sci. 2016, 7, 1156–1173. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Abboud, K.A.; Christou, G. MOF-like supramolecular network of Mn3 single-molecule magnets formed by extensive π-π stacking. Polyhedron 2016, 103, 150–156. [Google Scholar] [CrossRef]
- Shores, M.P.; Long, J.R. Tetracyanide-bridged divanadium complexes: Redox switching between strong antiferromagnetic and strong ferromagnetic coupling. J. Am. Chem. Soc. 2002, 124, 3512–3513. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.J.; Shores, M.P.; Bockrath, M.; Long, J.R.; Park, H. Kondo resonance in a single-molecule transistor. Nature 2002, 417, 725–729. [Google Scholar] [CrossRef]
- Atzori, M.; Benci, S.; Morra, E.; Tesi, L.; Chiesa, M.; Torre, R.; Sorace, L.; Sessoli, R. Structural effects on the spin dynamics of potential molecular qubits. Inorg. Chem. 2018, 57, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Chiesa, A.; Morra, E.; Chiesa, M.; Sorace, L.; Carreta, S.; Sessoli, R. A two-qubit molecular architecture for electron-mediated nuclear quantum simulation. Chem. Sci. 2018, 9, 6183–6192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doistau, B.; Cantin, J.-L.; Chamoreau, L.-M.; Marvaud, V.; Hasenknopf, B.; Vives, G. Mechanical switching of magnetic interaction by tweezers-type complex. Chem. Commun. 2015, 51, 12916–12919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, K.; Marsuda, K.; Irie, M. Photoswitching of the magnetic interaction between a copper(II) ion and a nitroxide radical by using a photochromic spin coupler. Chem. Eur. J. 2003, 9, 5605–5609. [Google Scholar] [CrossRef]
- Castellano, M.; Ruiz-García, R.; Cano, J.; Ferrando-Soria, J.; Pardo, E.; Fortéa-Pérez, F.R.; Stiriba, S.-E.; Barros, W.P.; Stumpf, H.O.; Cañadillas-Delgado, L.; et al. Metallosupramolecular approach toward multifunctional magnetic devices for molecular spintronics. Coord. Chem. Rev. 2015, 303, 110–138. [Google Scholar] [CrossRef]
- Castellano, M.; Ruiz-García, R.; Cano, J.; Ferrando-Soria, J.; Pardo, E.; Fortéa-Pérez, F.R.; Stiriba, S.-E.; Julve, M.; Lloret, F. Dicopper(II) metallacyclophanes as multifunctional magnetic devices: A joint experimental and computational study. Acc. Chem. Res. 2015, 48, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Molecular Wires. From Design to Properties. In Topics in Current Chemistry; Cola, L., Ed.; Springer: Berlin, Germany, 2005; Volume 257. [Google Scholar]
- Ruiz, E.; Rodríguez-Fortea, A.; Alvarez, S. Tailor-made strong exchange magnetic coupling through very long bridging ligands: Theoretical predictions. Inorg. Chem. 2003, 42, 4881–4891. [Google Scholar] [CrossRef]
- Ruiz, E.; Nunzi, F.; Alvarez, S. Magnetic communication through functionalized nanotubes: A theoretical study. Nano Lett. 2006, 6, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Nunzi, F.; Ruiz, E.; Cano, J.; Alvarez, S. Strong antiferromagnetic coupling at long distance through a ligand to metal charge transfer mechanism. J. Phys. Chem. C 2007, 111, 618–621. [Google Scholar] [CrossRef]
- Fabre, M.; Bonvoisin, J. Electronic and magnetic communication in mixed-valent and homovalent ruthenium complexes containing phenylcyanamide type bridging ligands. J. Am. Chem. Soc. 2007, 129, 1434–1444. [Google Scholar] [CrossRef] [Green Version]
- Paul, F.; Bondon, A.; Da Costa, G.; Malvolti, F.; Sinbandhit, S.; Cador, O.; Costuas, K.; Toupet, L.; Boillot, M.-L. Topological dependence of the magnetic exchange coupling in arylethynyl-bridged organometallic diradicals containing [(η2-dppe)(η5-C5Me5)FeIII]+ fragments. Inorg. Chem. 2009, 48, 10608–10624. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Castellano, M.; Yuste, C.; Lloret, F.; Julve, M.; Fabelo, O.; Ruiz-Pérez, C.; Stiriba, S.-E.; Ruiz-García, R.; Cano, J. Long-distance magnetic coupling in dinuclear copper(II) complexes with oligo-para-phenylenediamine bridging ligands. Inorg. Chim. Acta 2010, 363, 1666–1678. [Google Scholar] [CrossRef]
- Terencio, T.; Bastardis, R.; Suaud, N.; Maynau, D.; Bonvoisin, J.; Malrieu, J.P.; Calzado, C.J.; Guihery, N. Physical analysis of the through-ligand long-distance magnetic coupling: Spin-polarization versus Anderson mechanism. Phys. Chem. Chem. Phys. 2011, 13, 12314–12320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizawa, S.; Hasegawa, J.; Matsuda, K. Theoretical investigation of the β value of the phenylene and phenylene ethynylene units by evaluating exchange interaction between organic radicals. Chem. Phys. Lett. 2013, 555, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Cano, J.; Lloret, F.; Julve, M. Theoretical design of magnetic wires from acene and nanocorene derivatives. Dalton Trans. 2016, 45, 16700–16708. [Google Scholar] [CrossRef]
- Richert, S.; Cremers, J.; Kuprov, I.; Peeks, M.D.; Anderson, H.L.; Timmel, C.R. Constructive quantum interference in a bis-copper six-porphyrin nanoring. Nat. Commun. 2017, 8, 14842. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Brugh, A.M.; Rawson, J.; Therien, M.J.; Forbes, M.D.E. Alkyne-bridged multi[copper(II) prophyrin] structures: Nuances of orbital symmetry in long-range, through-bond mediated, isotropic spin exchange interactions. J. Am. Chem. Soc. 2017, 139, 9759–9762. [Google Scholar] [CrossRef]
- Starikova, A.A.; Minkin, V.I. Computational modeling of the dinuclear metal complexes with di-o-quinones comprising paramagnetic acene linker groups. Comput. Theor. Chem. 2018, 1138, 163–167. [Google Scholar] [CrossRef]
- Starikova, A.A.; Starikov, A.G.; Minyaer, R.M.; Boldyrev, A.I.; Minkin, V.I. Magnetic properties of acenes and their o-quinone derivatives: Computer simulation. Dokl. Chem. 2018, 478, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Ko, C.-H.; Brugh, A.M.; Bai, Y.; Forbes, M.D.E.; Therien, M.J. Topological, distance, and orbital symmetry effects on electronic spin-spin couplings in rigid molecular systems: Implications for long-distance spin-spin interactions. J. Phys. Chem. A 2020, 124, 7411–7415. [Google Scholar] [CrossRef]
- Davis, W.B.; Svec, W.A.; Ratner, M.A.; Wasielewski, M.R. Molecular-wire behaviour in p-phenylenevinylene oligomers. Nature 1998, 396, 60–63. [Google Scholar] [CrossRef]
- Davis, W.B.; Ratner, M.A.; Wasielewski, M.R. Conformational gating of long-distance electron transfer through wire-like bridges in donor-bridge-acceptor molecules. J. Am. Chem. Soc. 2001, 123, 7877–7886. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.A.; Ahrens, M.J.; Sinks, L.E.; Gusev, A.V.; Ratner, M.A.; Wasielewski, M.R. Making a molecular wire: Charge and spin transport through para-phenylene oligomers. J. Am. Chem. Soc. 2004, 126, 5577–5584. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.A.; Tauber, M.J.; Kelley, R.F.; Ahrens, M.J.; Ratner, M.A.; Wasielewski, M.R. Conformationally gated switching between superexchange and hopping within oligo-p-phenylene-based molecular wires. J. Am. Chem. Soc. 2005, 127, 11842–11850. [Google Scholar] [CrossRef]
- Kaliginedi, C.; Moreno-García, P.; Valkenier, H.; Hong, W.; García-Suárez, V.M.; Buiter, P.; Otten, J.L.H.; Hummelen, J.C.; Lambert, C.J.; Wandlowski, T. Correlations between molecular structure and single-junction conductance: A case study with oligo(phenylene-ethynylene)-type wires. J. Am. Chem. Soc. 2012, 134, 5262–5275. [Google Scholar] [CrossRef]
- Proppe, J.; Herrmann, C. Communication through molecular bridges: Different bridge orbital trends result in common property trends. J. Comput. Chem. 2014, 36, 201–209. [Google Scholar] [CrossRef]
- Herrmann, C. Electronic communication as a transferable property of molecular bridges? J. Phys. Chem. A 2019, 123, 10205–10223. [Google Scholar] [CrossRef] [Green Version]
- Fernández, I.; Ruiz, R.; Faus, J.; Julve, M.; Lloret, F.; Cano, J.; Ottenwaelder, X.; Journaux, Y.; Muñoz, M.C. Ferromagnetic coupling through spin polarization in a dinuclear copper(II) metallacyclophane. Angew. Chem. Int. Ed. 2001, 40, 3039–3042. [Google Scholar] [CrossRef]
- Pardo, E.; Ferrando-Soria, J.; Dul, M.C.; Lezcouëzec, R.; Journaux, Y.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cañadillas-Delgado, L.; et al. Oligo-m-phenyleneoxalamide copper(II) mesocates as electro-switchable ferromagnetic metal-organic wires. Chem. Eur. J. 2010, 16, 12838–12851. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Castellano, M.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Cañadillas-Delgado, L.; Li, Y.; et al. Redox switching of the antiferromagnetic coupling in permethylated dicopper(II) paracyclophanes. Chem. Commun. 2012, 48, 8401–8403. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Castellano, M.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Ruiz-Pérez, C.; Pasán, J.; Cañadillas-Delgado, L.; Armentano, D.; et al. Dicopper(II) metallacyclophanes with electroswitchable polymethyl-substituted para-phenylene spacers. Chem. Eur. J. 2013, 19, 12124–12137. [Google Scholar] [CrossRef]
- Malrieu, J.P.; Caballol, R.; Calzado, C.J.; de Graaf, C.; Guihéry, N. Magnetic interactions in molecules and highly correlated materials: Physical content, analytical derivation, and rigorous extraction of magnetic hamiltonians. Chem. Rev. 2014, 114, 429–492. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.S.; Vilela, R.S.; Valdo, A.K.; Martins, F.T.; García-España, E.; Inclán, M.; Cano, J.; Lloret, F.; Julve, M.; Stumpf, H.O.; et al. Dicopper(II) metallacyclophanes with N,N′-2,6-pyridinebis(oxamate): Solution study, synthesis, crystal structures, and magnetic properties. Inorg. Chem. 2016, 55, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.S.; Melo, W.D.C.; Kalinke, L.H.; Rabelo, R.; Valdo, A.K.; Da Silva, C.C.; Martins, F.T.; Amorós, P.; Lloret, F.; Julve, M.; et al. 2D and 3D mixed MII/CuII metal-organic frameworks (M = Ca and Sr) with N,N′-2,6-pyridinebis(oxamate) and oxalate: Preparation and magneto-structural study. Dalton Trans. 2018, 47, 11539–11553. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Journaux, Y.; De Munno, G.; Armentano, D. Multielectron transfer in a dicopper(II) anthraquinophane. Chem. Commun. 2013, 49, 3534–3536. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.; Barros, W.P.; Acosta, A.; Julve, M.; Lloret, F.; Li, Y.; Journaux, Y.; De Munno, G.; Armentano, D.; Ruiz-García, R.; et al. Dicopper(II) anthraquinophanes as multielectron reservoirs for oxidation and reduction: A joint experimental and computational study. Chem. Eur. J. 2014, 20, 13965–13975. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Faus, J.; Julve, M.; Lloret, F.; Muñoz, M.C.; Cano, J.; Ottenwaelder, X.; Journaux, Y.; Carrasco, R.; Blay, G.; et al. Long-range magnetic coupling through extended π-conjugated aromatic bridges in dinuclear copper(II) metallacyclophanes. J. Am. Chem. Soc. 2003, 125, 10770–10771. [Google Scholar] [CrossRef]
- Castellano, M.; Fortea-Pérez, F.R.; Stiriba, S.-E.; Julve, M.; Lloret, F.; Armentano, D.; De Munno, G.; Ruiz-García, R.; Cano, J. Very long-distance magnetic coupling in a dicopper(II) metallacyclophane with extended π-conjugated diphenylethyne bridges. Inorg. Chem. 2011, 50, 11279–11281. [Google Scholar] [CrossRef]
- Castellano, M.; Fortea-Pérez, F.R.; Bentama, A.; Stiriba, S.-E.; Julve, M.; Lloret, F.; De Munno, G.; Armentano, D.; Li, Y.; Ruiz-García, R.; et al. Dicopper(II) metallacyclophanes with oligo(p-phenylene-ethynylene) spacers: Experimental foundations and theoretical predictions on potential molecular magnetic wires. Inorg. Chem. 2013, 52, 7645–7657. [Google Scholar] [CrossRef]
- Pardo, E.; Carrasco, R.; Ruiz-García, R.; Julve, M.; Lloret, F.; Muñoz, M.C.; Journaux, Y.; Ruiz, E.; Cano, J. Structure and magnetism of dinuclear copper(II) metallacyclophanes with oligoacenebis(oxamate) bridging ligands: Theoretical predictions on wirelike magnetic coupling. J. Am. Chem. Soc. 2008, 130, 576–585. [Google Scholar] [CrossRef]
- Castellano, M.; Ferrando-Soria, J.; Pardo, E.; Julve, M.; Lloret, F.; Mathonière, C.; Pasán, J.; Ruiz-Pérez, C.; Cañadillas-Delgado, L.; Ruiz-García, R.; et al. Photoswitching of the antiferromagnetic coupling in an oxamato-based dicopper(II) anthracenophane. Chem. Commun. 2011, 47, 11035–11037. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.; Barros, W.P.; Ferrando-Soria, J.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Cañadillas-Delgado, L.; Ruiz-García, R.; Cano, J. Dicopper(II) metallacyclophanes with photoswitchable oligoacene spacers: A joint experimental and computational study on molecular magnetic photoswitches. J. Coord. Chem. 2018, 71, 675–692. [Google Scholar] [CrossRef]
- Coffman, R.E.; Buettner, G.R. A limit function for long-range ferromagnetic and antiferromagnetic superexchange. J. Phys. Chem. 1979, 83, 2387–2392. [Google Scholar] [CrossRef]
- Kahn, O. Dinuclear complexes with predictable magnetic properties. Angew. Chem. Int. Ed. Engl. 1985, 24, 834–850. [Google Scholar] [CrossRef]
- Castro, I.; Calatayud, M.L.; Yuste, C.; Castellano, M.; Ruiz-García, R.; Cano, J.; Faus, J.; Verdaguer, M.; Lloret, F. Dinuclear copper(II) complexes as testing ground for molecular magnetism theory. Polyhedron 2019, 169, 66–77. [Google Scholar] [CrossRef]
- Feringa, B.L. (Ed.) Molecular Switches; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Ellenbogan, J.C.; Love, J.C. Architectures for molecular electronic computers: 1. Logic structures and an adder designed from molecular electronic diodes. Proc. IEEE 2000, 88, 386–426. [Google Scholar] [CrossRef]
- Raymo, F.M. Digital processing and communication with molecular switches. Adv. Mater. 2002, 14, 401–414. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A.; Venturi, M. Molecular logic circuits. Chem. Phys. Chem. 2003, 4, 49–59. [Google Scholar] [CrossRef]
- De Silva, A.P.; McClenaghan, N.D. Molecular-scale logic gates. Chem. Eur. J. 2004, 10, 574–586. [Google Scholar] [CrossRef]
- De Silva, A.P. Molecular logic gets loaded. Nat. Mater. 2005, 4, 15–16. [Google Scholar] [CrossRef]
- Kume, S.; Nishihara, H. Metal-based photoswitches derived from photoisomerization. Struct. Bond. 2007, 123, 79–112. [Google Scholar]
- Trapp, O. Sensing on a molecular level-Chemistry at the interface of information technology. Angew. Chem. Int. Ed. 2008, 47, 8158–8160. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-Y.; Cui, L.; Li, J.; Yu, F.; Song, Y.; Zhang, Y.-Q.; Zuo, J.-L.; Kurmoo, M. Modulating single-moleule magnetic behavior of a dinuclear erbium(III) complex by solvent exchange. Inorg. Chem. 2017, 56, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhu, X.; Liu, P.; Su, S.; Hu, S.; Wen, Y.; Wu, X.; Sheng, T. Benzoquinone-bridged Co2 complexes with different magnetic anisotropy induced by solvent molecules. Dalton Trans. 2017, 46, 3435–3437. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Wylie, S.W.; Claus, B.L.; Horwitz, C.P.; Leychkis, Y.; Workman, J.M.; Marzec, A.J.; Clark, G.R.; Rickard, C.E.F.; Conklin, B.J.; Sellers, S.; et al. New magnetically coupled bimetallic complexes as potential building blocks for magnetic materials. Chem. Eur. J. 1998, 4, 2173–2181. [Google Scholar] [CrossRef]
- Fortier, S.; Le Roy, J.J.; Chen, C.-H.; Vieru, V.; Murugesu, M.; Chibotaru, L.F.; Mindiola, D.J.; Gaulton, K.G. A dinuclear cobalt complex featuring unprecedented anodic and cathodic redox switches for single-molecule magnet activity. J. Am. Chem. Soc. 2013, 135, 14670–14678. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Park, J.G.; Xiao, D.J.; Harris, T.D. An azophenine radical-bridged Fe2 single-molecule magnet with record magnetic exchange coupling. J. Am. Chem. Soc. 2013, 135, 16845–16848. [Google Scholar] [CrossRef]
- DeGayner, J.A.; Jeon, I.-R.; Harris, T.D. A series of tetraazalene radical-bridged M2 (M = CrIII, MnII, FeII, CoII) complexes with strong magnetic exchange coupling. Chem. Sci. 2015, 6, 6639–6648. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, S.; Wagner, A.; Kaifer, E.; Himmel, H.J. A valence tautomeric dinuclear copper tetrakisguanidine complex. Chem. Eur. J. 2016, 22, 10438–10445. [Google Scholar] [CrossRef]
- Yamasumi, K.; Nishimura, K.; Hisamune, Y.; Nagae, Y.; Uchiyama, T.; Kamitani, K.; Hirai, T.; Nishibori, M.; Mori, S.; Karasawa, S.; et al. Bis-copper(II)/π-radical multi-heterospin system with non-innocent doubly N-confused dioxohexaphyrin(1.1.1.1.1.0) ligand. Chem. Eur. J. 2017, 23, 15322–15326. [Google Scholar] [CrossRef]
- Di Piazza, E.; Boilleau, C.; Vacher, A.; Merahi, K.; Norel, L.; Costuas, K.; Roisnel, T.; Choua, S.; Turek, P.; Rigaut, S. Ruthenium carbon-rich group as a redox-switchable metal coupling unit in linear trinuclear complexes. Inorg. Chem. 2017, 56, 14540–14555. [Google Scholar] [CrossRef] [PubMed]
- Dolinar, B.S.; Gómez-Coca, S.; Alexandropoulos, D.I.; Dunbar, K.R. An air stable radical-bridged dysprosium single molecule magnet and its neutral counterpart: Redox switching of magnetic relaxation dynamics. Chem. Commun. 2017, 53, 2283–2286. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Layfield, R.A. Strong direct exchange coupling and single-molecule magnetism in indigo-bridged lanthanide dimers. Chem. Commun. 2017, 53, 3130–3133. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, K.; Irie, M. A diarylethene with two nitronyl nitroxides: Photoswitching of intramolecular magnetic interaction. J. Am. Chem. Soc. 2000, 122, 7195–7201. [Google Scholar] [CrossRef]
- Wang, L.-F.; Qiu, J.-Z.; Liu, J.-L.; Chen, Y.-C.; Jia, J.-H.; Jover, J.; Ruiz, E.; Tong, M.-L. Modulation of single-molecule magnet behaviour via photochemical [2+2] cycloaddition. Chem. Commun. 2015, 51, 15358–15361. [Google Scholar] [CrossRef] [Green Version]
- Salinas Uber, J.; Estrader, M.; Garcia, J.; Lloyd-Williams, P.; Sadurní, A.; Dengler, D.; Van Slageren, J.; Chilton, N.F.; Roubeau, O.; Teat, S.J.; et al. Molecules designed to contain two weakly coupled spins with a photoswitchable spacer. Chem. Eur. J. 2017, 23, 13648–13659. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-D.; Xu, Y.; Fan, K.; Bao, S.-S.; Kurmoo, M.; Zheng, L.-M. Reversible SC-SC transformation involving [4+4] cycloaddition of anthracene: A single-ion to single-molecule magnet and yellow-green to blue-white emission. Angew. Chem. Int. Ed. 2018, 57, 8577–8581. [Google Scholar] [CrossRef]
- Huang, X.-D.; Jia, J.-G.; Kurmoo, M.; Bao, S.-S.; Zheng, L.-M. Interplay of anthracene luminiscence and dysprosium magnetism by steric control of photodimerization. Dalton Trans. 2019, 48, 13769–13779. [Google Scholar] [CrossRef]
- Liu, J.-C.; Huang, X.-D.; Zou, Q.; Bao, S.-S.; Wang, X.-Z.; Ma, J.-Y.; Zheng, L.-M. Synergetic magnetic and luminiscence switching via solid state phase transitions of the dysprosium-dianthracene complex. J. Mater. Chem. C 2020, 8, 7369–7377. [Google Scholar] [CrossRef]
- Diefenbach, M.; Kim, K.S. Towards molecular magnetic switching with an electric bias. Angew. Chem. Int. Ed. 2007, 46, 7640–7643. [Google Scholar] [CrossRef]
- Baadji, N.; Piacenza, M.; Tugsuz, T.; Della Sala, F.; Maruccio, G.; Sanvito, S. Electrostatic spin crossover effect in polar magnetic molecules. Nat. Mater. 2009, 8, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Koley, S.; Chakrabarti, S. Large negative differential resistance and rectification from a donor-σ-acceptor molecule in the presence of dissimilar electrodes. Chem. Eur. J. 2018, 24, 5876–5882. [Google Scholar] [CrossRef]
- Do Pim, W.D.; Oliveira, W.X.C.; Ribeiro, M.A.; De Faria, E.N.; Teixeira, I.F.; Stumpf, H.O.; Lago, R.M.; Pereira, C.L.M.; Pinheiro, C.B.; Figueiredo-Júnior, J.C.D.; et al. A pH-triggered bistable copper(II) metallacycle as a metallacycle as a reversible emulsion switch for biphassic processes. Chem. Commun. 2013, 49, 10778–10780. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, T.T.; De Souza, T.E.; Do Pim, W.D.; De Almeida, L.D.; Do Nascimento, G.M.; García-España, E.; Inclán, M.; Julve, M.; Stumpf, H.O.; Oliveira, L.C.A.; et al. A hybrid catalyst for decontamination of organic pollutants based on a bifunctional dicopper(II) complex anchored over niobium oxyhydroxide. Appl. Catal. B Environ. 2017, 209, 339–345. [Google Scholar] [CrossRef]
- Do Pim, W.D.; Ribeiro-Santos, T.A.; Jardim, I.S.; Castro, M.C.M.; Braga, A.H.; Do Nascimento, G.M.; Binatti, I.; Stumpf, H.O.; Lorençon, E.; Araujo, M.H.; et al. Bistable copper(II) metallosurfactant as molecular machine for the preparation of hybrid silica-based porous materials. Mater. Des. 2018, 160, 876–885. [Google Scholar] [CrossRef]
- Liu, R.; Ke, S.-H.; Baranger, H.U.; Yang, W. Organometallic spintronics: Dicobaltocene switch. Nano Lett. 2005, 5, 1959–1962. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Hoffmann, R.; Strange, M.; Solomon, G.C. Close relation between quantum interference in molecular conductance and diradical existence. Proc. Nat. Acad. Sci. USA 2016, 113, E413–E419. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.; Tanifuji, N.; Yamaguchi, H.; Irie, M.; Matsuda, K. Photoswitching of conductance of diarylethene-Au nanoparticle network. Chem. Commun. 2007, 1355–1357. [Google Scholar] [CrossRef]
- Matsuda, K.; Yamaguchi, H.; Sakano, T.; Ikeda, M.; Tanifuji, N.; Irie, M. Conductance photoswitching of diarylethene nanoparticle network induced by photochromic reaction. J. Phys. Chem. C 2008, 112, 17005–17010. [Google Scholar] [CrossRef]
- Roldan, D.; Kaliginedi, V.; Cobo, S.; Kolivoska, V.; Bucher, C.; Hong, W.; Royal, G.; Wandlowski, T. Charge transport in photoswitchable dimethyldihydropyrene-type single-molecule junctions. J. Am. Chem. Soc. 2013, 135, 5974–5977. [Google Scholar] [CrossRef]
- Meng, F.; Hervault, Y.-M.; Shao, Q.; Hu, B.; Norel, L.; Rigaut, S.; Chen, X. Orthogonally modulated molecular transport junctions for resettable electronic logic gates. Nat. Commun. 2014, 5, 3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaripov, R.; Vavilova, E.; Khairuzhdinov, I.; Salikhov, K.; Voronkova, V.; Abdulmalic, M.A.; Meva, F.E.; Weheabby, S.; Rüffer, T.; Büchner, B.; et al. Tuning the spin coherence time of Cu(II)-(bis)oxamato and Cu(II)-(bis)oxamidato complexes by advanced ESR pulse protocols. Beilstein J. Nanotechnol. 2017, 8, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, R.; Stiriba, S.-E.; Cangussu, D.; Pereira, C.L.M.; Moliner, N.; Ruiz-García, R.; Cano, J.; Faus, J.; Journaux, Y.; Julve, M. When Molecular Magnetism Meets Supramolecular Chemistry: Multifunctional and Multiresponsive Dicopper(II) Metallacyclophanes as Proof-of-Concept for Single-Molecule Spintronics and Quantum Computing Technologies? Magnetochemistry 2020, 6, 69. https://doi.org/10.3390/magnetochemistry6040069
Rabelo R, Stiriba S-E, Cangussu D, Pereira CLM, Moliner N, Ruiz-García R, Cano J, Faus J, Journaux Y, Julve M. When Molecular Magnetism Meets Supramolecular Chemistry: Multifunctional and Multiresponsive Dicopper(II) Metallacyclophanes as Proof-of-Concept for Single-Molecule Spintronics and Quantum Computing Technologies? Magnetochemistry. 2020; 6(4):69. https://doi.org/10.3390/magnetochemistry6040069
Chicago/Turabian StyleRabelo, Renato, Salah-Eddine Stiriba, Danielle Cangussu, Cynthia L. M. Pereira, Nicolás Moliner, Rafael Ruiz-García, Joan Cano, Juan Faus, Yves Journaux, and Miguel Julve. 2020. "When Molecular Magnetism Meets Supramolecular Chemistry: Multifunctional and Multiresponsive Dicopper(II) Metallacyclophanes as Proof-of-Concept for Single-Molecule Spintronics and Quantum Computing Technologies?" Magnetochemistry 6, no. 4: 69. https://doi.org/10.3390/magnetochemistry6040069
APA StyleRabelo, R., Stiriba, S. -E., Cangussu, D., Pereira, C. L. M., Moliner, N., Ruiz-García, R., Cano, J., Faus, J., Journaux, Y., & Julve, M. (2020). When Molecular Magnetism Meets Supramolecular Chemistry: Multifunctional and Multiresponsive Dicopper(II) Metallacyclophanes as Proof-of-Concept for Single-Molecule Spintronics and Quantum Computing Technologies? Magnetochemistry, 6(4), 69. https://doi.org/10.3390/magnetochemistry6040069