Synthesis of a Doped α-Fe2O3/g-C3N4 Catalyst for High-Efficiency Degradation of Diazinon Contaminant from Liquid Wastes
Abstract
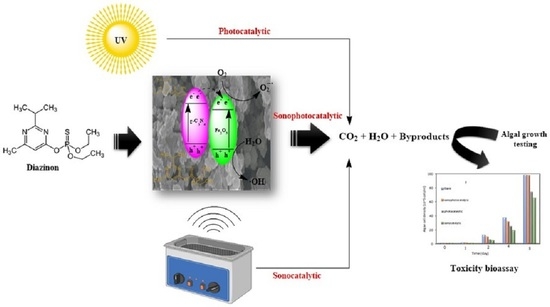
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of α-Fe2O3/g-C3N4 Composites
2.3. Catalytic Experiments
3. Results and Discussion
3.1. Characterization Results
3.2. Study of Effects of Operating Parameters
3.2.1. Ultrasonic Wave Frequency
3.2.2. Ultraviolet Light Intensity
3.2.3. Catalyst Dosage
3.2.4. Initial Diazinon Concentration
3.2.5. Comparative Study of Diazinon Degradation under Different Processes
3.2.6. Solution pH
3.3. Investigation of the Mineralization Behavior of Diazinon
3.4. Investigation of the Degradation Behavior of Diazinon
3.5. The Stability and Reusability of Fe2O3/g-C3N4
3.6. Scavenger Test
3.7. Determining Energy Consumption
3.8. Kinetic Degradation of Diazinon
3.9. Toxicological Experiments Using Algal Growth
3.10. Proposed Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Musawi, T.J.; McKay, G.; Rajiv, P.; Mengelizadeh, N.; Balarak, D. Efficient sonophotocatalytic degradation of acid blue 113 dye using a hybrid nanocomposite of CoFe2O4 nanoparticles loaded on multi-walled carbon nanotubes. J. Photochem. Photobiol. A Chem. 2022, 424, 113617. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Bagheri, N.; Kobya, M.; Şenocak, A.; Demirbas, E.; Karaoğlu, A.G. Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J. Ind. Eng. Chem. 2019, 77, 280–290. [Google Scholar] [CrossRef]
- Tariq, N.; Fatima, R.; Zulfiqar, S.; Rahman, A.; Warsi, M.F.; Shakir, I. Synthesis and characterization of MoO3/CoFe2O4 nanocomposite for photocatalytic applications. Ceram. Int. 2020, 46, 21596–21603. [Google Scholar] [CrossRef]
- Ayoubi-Feiz, B.; Mashhadizadeh, M.H.; Sheydaei, M. Degradation of diazinon by new hybrid nanocomposites N-TiO2/Graphene/Au and N-TiO2/Graphene/Ag using visible light photo-electro catalysis and photo-electro catalytic ozonation: Optimization and comparative study by Taguchi method. Sep. Purif. Technol. 2019, 211, 704–714. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Rajiv, P.; Mengelizadeh, N.; Sadat Arghavan, F. Photocatalytic efficiency of CuNiFe2O4 nanoparticles loaded on multi-walled carbon nanotubes as a novel photocatalyst for ampicillin degradation. J. Mol. Liq. 2021, 337, 116470. [Google Scholar] [CrossRef]
- Zhang, H.; Qiao, J.; Li, G.; Zhang, M.; Li, S.; Wang, J. Construction of coated Z-scheme Pd-BaZrO3@WO3 composite with enhanced sonocatalytic activity for diazinon degradation in aqueous solution. Sci. Total Environ. 2019, 663, 97–109. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Kassim, W.M.S.; Shahbaksh, S. Sonophotocatalytic degradation and operational parameters optimization of diazinon using magnetic cobalt-graphene nanocomposite as a catalyst. J. Water. Proc. Eng. 2022, 46, 102548. [Google Scholar] [CrossRef]
- Tabasideh, S.; Maleki, A.; Shahmoradi, B.; Ghahremani, E.; McKay, G. Sonophotocatalytic degradation of diazinon in aqueous solution using iron-doped TiO2 nanoparticles. Sep. Purif. Technol. 2017, 189, 186–192. [Google Scholar] [CrossRef]
- Vera, M.; Nyanhongo, G.S.; Guebitz, G.M.; Rivas, B.L. Polymeric microspheres as support to co-immobilized Agaricus bisporus and Trametes versicolor laccases and their application in diazinon degradation. Arab. J. Chem. 2020, 13, 4218–4227. [Google Scholar] [CrossRef]
- Yilmaz, M.; Mengelizadeh, N.; Saloot, M.K.; shahbaksh, S. Facile synthesis of Fe3O4/ZnO/GO photocatalysts for decolorization of acid blue 113 under solar, visible and UV lights. Mater. Sci. Semicond. Process. 2022, 144, 106593. [Google Scholar] [CrossRef]
- Rajiv, P.; Mengelizadeh, N.; McKay, G. Photocatalytic degradation of ciprofloxacin with Fe2O3 nanoparticles loaded on graphitic carbon nitride: Mineralisation, degradation mechanism and toxicity assessment. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Balarak, D.; Mostafapour, F.K. Photocatalytic degradation of amoxicillin using UV/Synthesized NiO from pharmaceutical wastewater. Indones. J. Chem. 2019, 19, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Hanh, N.T.; Van Thuan, D.; Khai, N.M.; Thuy, P.T.; Hang, T.T.M.; Vy, N.H.T.; Van Noi, N.; Tran, D.T.; Pham, T.D.; Truc, N.T.T.; et al. Synthesis of Co3O4 coated on N,S doped TiO2 for novel photocatalytic degradation of toxic organic pollutant in aqueous environment. Ceram. Int. 2020, 46, 21610–21616. [Google Scholar] [CrossRef]
- Borji, S.H.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Javadi, A.H. Investigation of photocatalytic degradation of phenol by Fe (III)-doped TiO2 and TiO2 nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskandari, N.; Nabiyouni, G.; Masoumi, S.; Ghanbari, D. Preparation of a new magnetic and photo-catalyst CoFe2O4–SrTiO3 perovskite nanocomposite for photo-degradation of toxic dyes under short time visible irradiation. Compos. Part B Eng. 2019, 176, 107343. [Google Scholar] [CrossRef]
- Balarak, D.; Mengelizadeh, N.; Rajiv, P.; Chandrika, K. Photocatalytic degradation of amoxicillin from aqueous solutions by titanium dioxide nanoparticles loaded on graphene oxide. Environ. Sci. Pollut. Res. 2021, 28, 49743–49754. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J. Z-Scheme 2D/2D α-Fe2O3/g-C3N4 heterojunction for photocatalytic oxidation of nitric oxide. Appl. Catal. B Environ. 2021, 280, 119409. [Google Scholar] [CrossRef]
- Wang, M.; Cui, S.; Yang, X.; Bi, W. Synthesis of g-C3N4/Fe3O4 nanocomposites and application as a new sorbent for solid phase extraction of polycyclic aromatic hydrocarbons in water samples. Talanta 2015, 132, 922–928. [Google Scholar] [CrossRef]
- John, P.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Enhanced photocatalytic performance of visible light driven TiO2/g-C3N4 for degradation of diclofenac in aqueous solution. Environ. Technol. Innov. 2021, 22, 101412. [Google Scholar] [CrossRef]
- Zhong, Q.; Lan, H.; Zhang, M.; Zhu, H.; Bu, M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Singh, J.; Basu, S. Synthesis of mesoporous magnetic Fe2O3/g-C3N4 monoliths for Rhodamine B removal. Microporous Mesoporous Mater. 2020, 303, 110299. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, C.; Mei, J.; Yang, S.; Wong, P.K. Faster electron injection and higher interface reactivity in g-C3N4/Fe2O3 nanohybrid for efficient photo-Fenton-like activity toward antibiotics degradation. Environ. Res. 2021, 195, 110842. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shi, Y.; Tao, Y.; Zhang, H. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4/Fe2O3 heterojunction. Chem. Eng. J. 2019, 359, 933–943. [Google Scholar] [CrossRef]
- Demir, M.; Taymaz, B.H.; Sarıbel, M.; Kamış, H. Photocatalytic Degradation of Organic Dyes with Magnetically Separable PANI/Fe3O4 Composite under Both UV and Visible-light Irradiation. ChemistrySelect 2022, 7, e202103787. [Google Scholar] [CrossRef]
- Hu, F.; Sun, S.; Xu, H.; Li, M.; Hao, X.; Shao, G.; Wang, H.; Chen, D.; Lu, H.; Zhang, R. Investigation on g-C3N4/rGO/TiO2 nanocomposite with enhanced photocatalytic degradation performance. J. Phys. Chem. Solids 2021, 156, 110181. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Noh, M.F.M.; Ita, N.S.H.M.; Mohamed, N.A.; Nasir, S.N.F.M.; Mumthas, I.N.N. Nanostructure-assisted charge transfer in alpha-Fe2O3/g-C3N4 heterojunctions for efficient and highly stable photoelectrochemical water splitting. Dalton Trans. 2020, 49, 11317–11328. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, S.; Jun, B.-M.; Prabhu, S.M.; Elanchezhiyan, S.S.; Ok, Y.S.; Meenakshi, S.; Park, C.M. Enhanced sonophotocatalytic degradation of bisphenol A using bimetal sulfide-intercalated MXenes, 2D/2D nanocomposite. Sep. Purif. Technol. 2020, 250, 117178. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Taghavi, M.; Shehu, Z.; Balarak, D. Capability of copper-nickel ferrite nanoparticles loaded onto multi-walled carbon nanotubes to degrade acid blue 113 dye in the sonophotocatalytic treatment process. Environ. Sci. Pollut. Res. 2022, 29, 51703–51716. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Rajiv, P.; Mengelizadeh, N.; Mohammed, I.A.; Balarak, D. Development of sonophotocatalytic process for degradation of acid orange 7 dye by using titanium dioxide nanoparticles/graphene oxide nanocomposite as a catalyst. J. Environ. Manag. 2021, 292, 112777. [Google Scholar] [CrossRef]
- Dhanasekar, M.; Ratha, S.; Rout, C.S.; Bhat, S.V. Efficient sono-photocatalytic degradation of methylene blue using nickel molybdate nanosheets under diffused sunlight. J. Environ. Chem. Eng. 2017, 5, 2997–3004. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Ahmed, W.; Elhissi, A.; Khalid, N.R. Photocatalytic, sonocatalytic and sonophotocatalytic degradation of Rhodamine B using ZnO/CNTs composites photocatalysts. Ultrason. Sonochem. 2014, 21, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2019, 50, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.R.; Sarode, K.M.; Nerkar, D.M.; Patil, U.D.; Bachhav, S.G.; Sonawane, U.S.; Paliwal, N. Sonocatalytic Degradation of Methylene Blue by MoS2-RGO Nanocomposites. Russ. J. Phys. Chem. A 2021, 95, 2530–2537. [Google Scholar] [CrossRef]
- Kakavandi, B.; Bahari, N.; Rezaei Kalantary, R.; Fard, E.D. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef]
- Liu, T.; He, F.W.; Zhang, Y.Q. Synergistic Degradation of Acid Scarlet Dyeing Wastewater by the Ultrasound/Fenton Method. Appl. Mech. Mater. 2013, 448–453, 34–37. [Google Scholar] [CrossRef]
- Lim, M.; Son, Y.; Khim, J. Frequency effects on the sonochemical degradation of chlorinated compounds. Ultrason. Sonochem. 2011, 18, 460–465. [Google Scholar] [CrossRef]
- He, Y.; Grieser, F.; Ashokkumar, M. The mechanism of sonophotocatalytic degradation of methyl orange and its products in aqueous solutions. Ultrason. Sonochem. 2011, 18, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Benomara, A.; Guenfoud, F.; Mokhtari, M.; Boudjemaa, A. Sonolytic, sonocatalytic and sonophotocatalytic degradation of a methyl violet 2B using iron-based catalyst. React. Kinet. Mech. Catal. 2021, 132, 513–528. [Google Scholar] [CrossRef]
- Ghosh, S.; Samanta, M.; Sen, D.; Sarkar, S.; Sarkar, S.; Chattopadhyay, K.K. Photocatalytic and sonocatalytic dye degradation by sulfur vacancy rich ZnS nanopowder. J. Nanoparticle Res. 2021, 23, 160. [Google Scholar] [CrossRef]
- Nasirian, M.; Mehrvar, M. Modification of TiO2 to enhance photocatalytic degradation of organics in aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 4072–4082. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Khan, M.A.N.; Siddique, M.; Wahid, F.; Khan, R. Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light. Ultrason. Sonochem. 2015, 26, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Karimi, A.; Zarei, M.; Joo, S.W. Eu-doped ZnO nanoparticles: Sonochemical synthesis, characterization, and sonocatalytic application. Ultrason. Sonochem. 2020, 67, 102822. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Liang, C.; Chen, J.-H. Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere 2011, 82, 1168–1172. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Priya, A.; Madhavan, J.; Michael, R.J.; Ashokkumar, M. Photocatalytic and photoelectrochemical studies of visible-light active α-Fe2O3– g-C3N4 nanocomposites. RSC Adv. 2014, 4, 38222–38229. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Cao, J.; Tang, Q.; Li, M.; Kang, P.; Shi, C.; Ma, M. Ultrasonic-assisted synthesis of 2D α-Fe2O3@ g-C3N4 composite with excellent visible light photocatalytic activity. Catalysts 2018, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Fan, Y.; Wu, R.; Huo, Y.; Wu, H.; Wang, F.; Xu, X. Novel magnetic gC3N4/α-Fe2O3/Fe3O4 composite for the very effective visible-light-Fenton degradation of Orange II. RSC Adv. 2018, 8, 5180–5188. [Google Scholar] [CrossRef] [Green Version]
- Eshaq, G.; Wang, S.; Sun, H.; Sillanpaa, M. Superior performance of FeVO4@CeO2 uniform core-shell nanostructures in heterogeneous Fenton-sonophotocatalytic degradation of 4-nitrophenol. J. Hazard. Mater. 2020, 382, 121059. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Gondal, M.A.; Drmosh, Q.A.; Yamani, Z.H.; Al-yamani, A. Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 407–412. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Sathishkumar, K.; Mohebi, S. Preparation of CuFe2O4/montmorillonite nanocomposite and explaining its performance in the sonophotocatalytic degradation process for ciprofloxacin. Colloid Interface Sci. Commun. 2021, 45, 100532. [Google Scholar] [CrossRef]
- Bāzghale, Ā.A.; Mohammad-khāh, A. Sonocatalytic decolorization of methylene blue from aqueous media by La: ZnO/GO nanocomposites. Res. Chem. Intermed. 2019, 45, 1985–2005. [Google Scholar] [CrossRef]
- Bampos, G.; Frontistis, Z. Sonocatalytic degradation of butylparaben in aqueous phase over Pd/C nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 11905–11919. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.; Malakootian, M.; Heidari, M.R.; Asadzadeh, S.N. CoFe2O4@Methylcelloluse as a New Magnetic Nano Biocomposite for Sonocatalytic Degradation of Reactive Blue 19. J. Polym. Environ. 2021, 29, 2660–2675. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Lee, R.K.L. Enhancement of sonocatalytic degradation of organic dye by using titanium dioxide (TiO2)/activated carbon (AC) derived from oil palm empty fruit bunch. Environ. Sci. Pollut. Res. 2020, 27, 34638–34652. [Google Scholar] [CrossRef] [PubMed]
- Dbira, S.; Bensalah, N.; Zagho, M.M.; Ennahaoui, M.; Bedoui, A. Oxidative Degradation of Tannic Acid in Aqueous Solution by UV/S2O82− and UV/H2O2/Fe2+ Processes: A Comparative Study. Appl. Sci. 2019, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Lee, B.-K. Photocatalytic reduction of carbon dioxide to methanol using nickel-loaded TiO2 supported on activated carbon fiber. Catal. Today 2017, 298, 158–167. [Google Scholar] [CrossRef]
- Lin, S.-H.; Chiou, C.-H.; Chang, C.-K.; Juang, R.-S. Photocatalytic degradation of phenol on different phases of TiO2 particles in aqueous suspensions under UV irradiation. J. Environ. Manag. 2011, 92, 3098–3104. [Google Scholar] [CrossRef]
- Kumar, K.; Selvam, B. Influence of operational parameters on photomineralization of Acid Black 1 with ZnO. Desalination Water Treat. 2010, 24, 132–139. [Google Scholar]
- Arghavan, F.S.; Al-Musawi, T.J.; Allahyari, E.; Moslehi, M.H.; Nasseh, N.; Hossein Panahi, A. Complete degradation of tamoxifen using FeNi3@SiO2@ZnO as a photocatalyst with UV light irradiation: A study on the degradation process and sensitivity analysis using ANN tool. Mater. Sci. Semicond. Process. 2021, 128, 105725. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Publishing: Paris, French, 2011. [Google Scholar]
- Moradi, M.; Kakavandi, B.; Bahadoran, A.; Giannakis, S.; Dehghanifard, E. Intensification of persulfate-mediated elimination of bisphenol A by a spinel cobalt ferrite-anchored g-C3N4 S-scheme photocatalyst: Catalytic synergies and mechanistic interpretation. Sep. Purif. Technol. 2022, 285, 120313. [Google Scholar] [CrossRef]
- Sun, S.; Ji, C.; Wu, L.; Chi, S.; Qu, R.; Li, Y.; Lu, Y.; Sun, C.; Xue, Z. Facile one-pot construction of α-Fe2O3/g-C3N4 heterojunction for arsenic removal by synchronous visible light catalysis oxidation and adsorption. Mater. Chem. Phy. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A.; Kirillov, A.M. Instantaneous sonophotocatalytic degradation of tetracycline over NU-1000@ ZnIn2S4 core–shell nanorods as a robust and eco-friendly catalyst. Inorg. Chem. 2021, 60, 9660–9672. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A comprehensive review on sonocatalytic, photocatalytic, and sonophotocatalytic processes for the degradation of antibiotics in water: Synergistic mechanism and degradation pathway. Chem. Eng. J. 2021, 413, 127412. [Google Scholar] [CrossRef]
- Liu, G.; Ji, J.; Huang, H.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; Fang, R.; He, M.; Liu, S.; et al. UV/H2O2: An efficient aqueous advanced oxidation process for VOCs removal. Chem. Eng. J. 2017, 324, 44–50. [Google Scholar] [CrossRef]








| Pollutant | Catalyst Dose (g/L) | Time (min) | Method | Degradation (%) | Ref. |
|---|---|---|---|---|---|
| Bisphenol A (0.5 mM) | 0.5 | 60 | Persulfate activation | 92.2 | [23] |
| Direct red 81 (0.05 mM) | 1.25 | 120 | Photocatalytic | ≈80 | [46] |
| Rhodamine B (10 mg/L) | 1 | 120 | Photocatalytic | 90 | [47] |
| Orange II (50 mg/L) | - | 60 | Photo-Fenton | ≈80 | [48] |
| DZN (100 mg/L) | 0.4 | 60 | Sonophotocatalytic | 100 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Musawi, T.J.; Asgariyan, R.; Yilmaz, M.; Mengelizadeh, N.; Asghari, A.; Balarak, D.; Darvishmotevall, M. Synthesis of a Doped α-Fe2O3/g-C3N4 Catalyst for High-Efficiency Degradation of Diazinon Contaminant from Liquid Wastes. Magnetochemistry 2022, 8, 137. https://doi.org/10.3390/magnetochemistry8110137
Al-Musawi TJ, Asgariyan R, Yilmaz M, Mengelizadeh N, Asghari A, Balarak D, Darvishmotevall M. Synthesis of a Doped α-Fe2O3/g-C3N4 Catalyst for High-Efficiency Degradation of Diazinon Contaminant from Liquid Wastes. Magnetochemistry. 2022; 8(11):137. https://doi.org/10.3390/magnetochemistry8110137
Chicago/Turabian StyleAl-Musawi, Tariq J., Rasoul Asgariyan, Murat Yilmaz, Nezamaddin Mengelizadeh, Abolfazl Asghari, Davoud Balarak, and Mohammad Darvishmotevall. 2022. "Synthesis of a Doped α-Fe2O3/g-C3N4 Catalyst for High-Efficiency Degradation of Diazinon Contaminant from Liquid Wastes" Magnetochemistry 8, no. 11: 137. https://doi.org/10.3390/magnetochemistry8110137
APA StyleAl-Musawi, T. J., Asgariyan, R., Yilmaz, M., Mengelizadeh, N., Asghari, A., Balarak, D., & Darvishmotevall, M. (2022). Synthesis of a Doped α-Fe2O3/g-C3N4 Catalyst for High-Efficiency Degradation of Diazinon Contaminant from Liquid Wastes. Magnetochemistry, 8(11), 137. https://doi.org/10.3390/magnetochemistry8110137