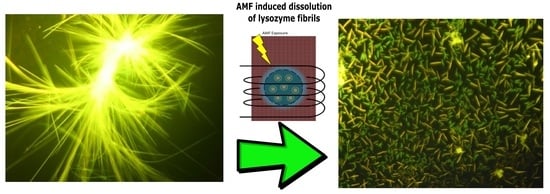
Dissolution of Lysozyme Amyloid Fibrils Using Magnetic Nanoparticles in an Alternating Magnetic Field: Design of an Effective Treatment for Cutaneous Amyloidosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amyloid Fibrils Preparation
2.3. Thioflavin T Binding Assays
2.4. Fluorescence Microscopy
2.5. CR Binding Assay
2.6. Application of Alternating Magnetic Field (AMF)
2.7. Measurement of Magnetization Curve
2.8. Calculation of Fractal Dimension
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Šimaljaková, M.; Buchvald, D. Dermatovenereology; Comenius University Press: Bratislava, Slovakia, 2019. [Google Scholar]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.; E Balch, W.; Kelly, J.W. Functional Amyloid Formation within Mammalian Tissue. PLOS Biol. 2005, 4, e6. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2015, 387, 2641–2654. [Google Scholar] [CrossRef]
- Dogan, A. Amyloidosis: Insights from Proteomics. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 277–304. [Google Scholar] [CrossRef]
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef]
- Hazenberg, B.P. Amyloidosis: A clinical overview. Rheum. Dis. Clin. N. Am. 2013, 39, 323–345. [Google Scholar] [CrossRef] [Green Version]
- Alraawi, Z.; Banerjee, N.; Mohanty, S.; Kumar, T.K.S. Amyloidogenesis: What Do We Know So Far? Int. J. Mol. Sci. 2022, 23, 13970. [Google Scholar] [CrossRef]
- Hamie, L.; Haddad, I.; Nasser, N.; Kurban, M.; Abbas, O. Primary Localized Cutaneous Amyloidosis of Keratinocyte Origin: An Update with Emphasis on Atypical Clinical Variants. Am. J. Clin. Dermatol. 2021, 22, 667–680. [Google Scholar] [CrossRef]
- Low, K.J.Y.; Venkatraman, A.; Mehta, J.S.; Pervushin, K. Molecular mechanisms of amyloid disaggregation. J. Adv. Res. 2021, 36, 113–132. [Google Scholar] [CrossRef]
- Almeida, Z.L.; Brito, R.M.M. Amyloid Disassembly: What Can We Learn from Chaperones? Biomedicines 2022, 10, 3276. [Google Scholar] [CrossRef]
- Almeida, Z.L.; Brito, R.M.M. Structure and Aggregation Mechanisms in Amyloids. Molecules 2020, 25, 1195. [Google Scholar] [CrossRef] [Green Version]
- Picone, P.; Sanfilippo, T.; Vasto, S.; Baldassano, S.; Guggino, R.; Nuzzo, D.; Bulone, D.; Biagio, P.L.S.; Muscolino, E.; Monastero, R.; et al. From Small Peptides to Large Proteins against Alzheimer’sDisease. Biomolecules 2022, 12, 1344. [Google Scholar] [CrossRef]
- Shao, X.; Yan, C.; Wang, C.; Cao, Y.; Zhou, Y.; Guan, P.; Hu, X.; Zhu, W.; Ding, S. Advanced nanomaterials for modulating Alzheimer’s related amyloid aggregation. Nanoscale Adv. 2022, 5, 46–80. [Google Scholar] [CrossRef]
- Liang, C.-Q.; Li, Y.-M. Peptides for disrupting and degrading amyloids. Curr. Opin. Chem. Biol. 2021, 64, 124–130. [Google Scholar] [CrossRef]
- Huang, Y.; Chang, Y.; Liu, L.; Wang, J. Nanomaterials for Modulating the Aggregation of β-Amyloid Peptides. Molecules 2021, 26, 4301. [Google Scholar] [CrossRef]
- Andrei, M.; Wang, J.C. Cutaneous light chain amyloidosis with multiple myeloma: A concise review. Hematol. Stem Cell Ther. 2018, 12, 71–81. [Google Scholar] [CrossRef]
- Wenson, S.F.; Jessup, C.J.; Johnson, M.M.; Cohen, L.M.; Mahmoodi, M. Primary cutaneous amyloidosis of the external ear: A clinicopathological and immunohistochemical study of 17 cases. J. Cutan. Pathol. 2011, 39, 263–269. [Google Scholar] [CrossRef]
- Fernandez-Flores, A. Cutaneous Amyloidosis: A Concept Review. Am. J. Dermatopathol. 2012, 34, 1–17. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.-M.; Vogt, T.; Landthaler, M.; Schroeder, J.; Babilas, P. Cutaneous amyloidoses and systemic amyloidoses with cutaneous involvement. Eur. J. Dermatol. 2010, 20, 152–160. [Google Scholar] [CrossRef]
- Yoneyama, K.; Tochigi, N.; Oikawa, A.; Shinkai, H.; Utani, A. Primary Localized Cutaneous Nodular Amyloidosis in a Patient with Sjögren’s Syndrome: A Review of the Literature. J. Dermatol. 2005, 32, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Weidner, T.; Illing, T.; Elsner, P. Primary Localized Cutaneous Amyloidosis: A Systematic Treatment Review. Am. J. Clin. Dermatol. 2017, 18, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Ahramiyanpour, N.; Akbari, Z.; Sarasyabi, M.S.; Aflatoonian, M.; Saki, N.; Shafie’ei, M. The therapeutic role of lasers in pri-mary localized cutaneous amyloidosis: A systematic review. Lasers Med. Sci. 2022, 37, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.S.; Chandra, N. Lysozyme. Rev. Med. Interne. 2011, 84, 63–111. [Google Scholar] [CrossRef]
- Scafi, M.; Valleix, S.; Benyamine, A.; Jean, E.; Harlé, J.-R.; Rossi, P.; Daniel, L.; Schleinitz, N.; Granel, B. L’amylose à lysozyme: A model protein for amyloid research. Adv. Protein Chem. Struct. Biol. 2018, 40, 323–329. [Google Scholar] [CrossRef]
- Pleyer, C.; Flesche, J.; Saeed, F. Lysozyme amyloidosis–A case report and review of the literature. Clin. Nephrol. Case Stud. 2015, 3, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Booth, D.R.; Sunde, M.; Bellotti, V.; Robinson, C.V.; Hutchinson, W.L.; Fraser, P.E.; Hawkins, P.N.; Dobson, C.M.; Radford, S.E.; Blake, C.C.; et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 1997, 385, 787–793. [Google Scholar] [CrossRef]
- Gombos, J.; Balejcikova, L.; Kopcansky, P.; Batkova, M.; Siposova, K.; Kovac, J.; Zolochevska, K.; Safarik, I.; Lokajova, A.; Garamus, V.M.; et al. Destruction of Lysozyme Amyloid Fibrils Induced by Magnetoferritin and Reconstructed Ferritin. Int. J. Mol. Sci. 2022, 23, 13926. [Google Scholar] [CrossRef]
- Bellova, A.; Bystrenova, E.; Koneracka, M.; Kopcansky, P.; Valle, F.; Tomasovicova, N.; Timko, M.; Bagelova, J.; Biscarini, F.; Gazova, Z. Effect of Fe3O4 magnetic nanoparticles on lysozyme amyloid aggregation. Nanotechnology 2010, 21, 065103. [Google Scholar] [CrossRef]
- Solin, N. Amyloid-like fibrils labeled with magnetic nanoparticles. Biomol. Concepts 2013, 4, 425–432. [Google Scholar] [CrossRef]
- Majorosova, J.; Petrenko, V.I.; Siposova, K.; Timko, M.; Tomasovicova, N.; Garamus, V.M.; Koralewski, M.; Avdeev, M.V.; Leszczynski, B.; Jurga, S.; et al. On the adsorption of magnetite nanoparticles on lysozyme amyloid fibrils. Colloids Surfaces B Biointerfaces 2016, 146, 794–800. [Google Scholar] [CrossRef]
- Antosova, A.; Gancar, M.; Bednarikova, Z.; Marek, J.; Zahn, D.; Dutz, S.; Gazova, Z. Surface-modified magnetite nanoparticles affect lysozyme amyloid fibrillization. Biochim. et Biophys. Acta BBA Gen. Subj. 2021, 1865, 129941. [Google Scholar] [CrossRef]
- Lee, W.; Jung, H.; Son, M.; Lee, H.; Kwak, T.J.; Lee, G.; Kim, C.H.; Lee, S.W.; Yoon, D.S. Characterization of the regrowth behavior of amyloid-like fragmented fibrils decomposed by ultrasonic treatment. RSC Adv. 2014, 4, 56561–56566. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Romero, G.; Christiansen, M.G.; Chen, R.; Ellison, R.; O’Malley, T.T.; Froriep, U.P.; Walsh, D.M.; Anikeeva, P. Targeted Magnetic Nanoparticles for Remote Magnetothermal Disruption of Amyloid-β Aggregates. Adv. Health Mater. 2015, 4, 2100–2109. [Google Scholar] [CrossRef]
- Dyne, E.; Prakash, P.S.; Li, J.; Yu, B.; Schmidt, T.-L.; Huang, S.; Kim, M.-H. Mild magnetic nanoparticle hyperthermia promotes the disaggregation and microglia-mediated clearance of beta-amyloid plaques. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102397. [Google Scholar] [CrossRef]
- Bastús, N.G.; Kogan, M.J.; Amigo, R.; Grillo-Bosch, D.; Araya, E.; Turiel, A.; Labarta, A.; Giralt, E.; Puntes, V.F. Gold nanoparticles for selective and remote heating of β-amyloid protein aggregates. Mater. Sci. Eng. C 2007, 27, 1236–1240. [Google Scholar] [CrossRef]
- Triulzi, R.C.; Dai, Q.; Zou, J.; Leblanc, R.M.; Gu, Q.; Orbulescu, J.; Huo, Q. Photothermal ablation of amyloid aggregates by gold nanoparticles. Colloids Surf. B Biointerfaces 2008, 63, 200–208. [Google Scholar] [CrossRef]
- Babincová, M. Microwave induced likage of magnetoliposomes. Possible clinical implications. Bioelectrochem. Bioenerg. 1993, 32, 187–189. [Google Scholar] [CrossRef]
- Babincová, M.; Vrbovská, H.; Sourivong, P.; Babinec, P.; Durdík, Š. Application of Albumin-embedded Magnetic Nanoheaters for Release of Etoposide in Integrated Chemotherapy and Hyperthermia of U87-MG Glioma Cells. Anticancer. Res. 2018, 38, 2683–2690. [Google Scholar] [CrossRef]
- Caizer, C. Optimization Study on Specific Loss Power in Superparamagnetic Hyperthermia with Magnetite Nanoparticles for High Efficiency in Alternative Cancer Therapy. Nanomaterials 2020, 11, 40. [Google Scholar] [CrossRef]
- Zahn, D.; Landers, J.; Buchwald, J.; Diegel, M.; Salamon, S.; Müller, R.; Köhler, M.; Ecke, G.; Wende, H.; Dutz, S. Ferrimagnetic Large Single Domain Iron Oxide Nanoparticles for Hyperthermia Applications. Nanomaterials 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Babincová, N.; Sourivong, P.; Babinec, P.; Bergemann, C.; Babincová, M.; Durdík, S. Applications of magnetoliposomes with encapsulated doxorubicin for integrated chemotherapy and hyperthermia of rat C6 glioma. Z. Nat. C 2018, 73, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.K. Spectroscopic Characterization of Amyloid Fibril Formation by Lysozyme. J. Chem. Educ. 2014, 91, 730–733. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39, 1495–1506. [Google Scholar] [CrossRef] [Green Version]
- Howie, A.J. “Green (or apple-green) birefringence” of Congo red-stained amyloid. Amyloid 2015, 22, 205–206. [Google Scholar] [CrossRef]
- Howie, A.J.; Brewer, D.B. Optical properties of amyloid stained by Congo red: History and mechanisms. Micron 2008, 40, 285–301. [Google Scholar] [CrossRef]
- Howie, A.J.; Brewer, D.B.; Howell, D.; Jones, A.P. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab. Investig. 2008, 88, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Zmeskal, O.; Bzatek, T.; Nezadal, M.; HarFA: Harmonic and Fractal Image Analyser. Software for Determination of Fractal Dimension. Available online: http://www.fch.vutbr.cz/lectures/imagesci (accessed on 19 March 2017).
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2009, 256, 3093–3097. [Google Scholar] [CrossRef]
- Tombácz, E.; Bica, D.; Hajdú, A.; Illés, E.; Majzik, A.; Vékás, L. Surfactant double layer stabilized magnetic nanofluids for bio-medical application. J. Phys. Condens. Matter 2008, 20, 204103. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Y.; He, B.; Zeng, X.; Lai, K.; Gu, Z. Effect of sodium oleate as a buffer on the synthesis of superparamagnetic magnetite colloids. J. Colloid Interface Sci. 2010, 347, 1–7. [Google Scholar] [CrossRef]
- Bateer, B.; Qu, Y.; Meng, X.; Tian, C.; Du, S.; Wang, R.; Pan, K.; Fu, H. Preparation and magnetic performance of the magnetic fluid stabilized by bi-surfactant. J. Magn. Magn. Mater. 2012, 332, 151–156. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. Part A 2006, 80A, 333–341. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, BME-31, 70–75. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Garaio, E.; Sandre, O.; Collantes, J.-M.; Garcia, J.A.; Mornet, S.; Plazaola, F. Specific absorption rate dependence on temperature in magnetic field hyperthermia measured by dynamic hysteresis losses (ac magnetometry). Nanotechnology 2014, 26, 015704. [Google Scholar] [CrossRef] [Green Version]
- Makshakova, O.; Bogdanova, L.; Faizullin, D.; Khaibrakhmanova, D.; Ziganshina, S.; Ermakova, E.; Zuev, Y.; Sedov, I. The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics 2023, 15, 624. [Google Scholar] [CrossRef]
- Capocefalo, A.; Deckert-Gaudig, T.; Brasili, F.; Postorino, P.; Deckert, V. Unveiling the interaction of protein fibrils with gold nanoparticles by plasmon enhanced nano-spectroscopy. Nanoscale 2021, 13, 14469–14479. [Google Scholar] [CrossRef] [PubMed]
- Barbalinardo, M.; Antosova, A.; Gambucci, M.; Bednarikova, Z.; Albonetti, C.; Valle, F.; Sassi, P.; Latterini, L.; Gazova, Z.; Bystrenova, E. Effect of metallic nanoparticles on amyloid fibrils and their influence to neural cell toxicity. Nano Res. 2020, 13, 1081–1089. [Google Scholar] [CrossRef]
- Ban, D.K.; Paul, S. Functionalized gold and silver nanoparticles modulate amyloid fibrillation, defibrillation and cytotoxicity of lysozyme via altering protein surface character. Appl. Surf. Sci. 2018, 473, 373–385. [Google Scholar] [CrossRef]
- Mari, E.; Ricci, C.; Pieraccini, S.; Spinozzi, F.; Mariani, P.; Ortore, M.G. Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils. Life 2020, 10, 60. [Google Scholar] [CrossRef]
- Mastrella, L.; Moretti, P.; Pieraccini, S.; Magi, S.; Piccirillo, S.; Ortore, M.G. Taurine Stabilizing Effect on Lysozyme. Life 2022, 12, 133. [Google Scholar] [CrossRef]
- Skaat, H.; Sorci, M.; Belfort, G.; Margel, S. Effect of maghemite nanoparticles on insulin amyloid fibril formation: Selective labeling, kinetics, and fibril removal by a magnetic field. J. Biomed. Mater. Res. Part A 2008, 91A, 342–351. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Debelouchina, G.T.; Bayro, M.J.; Clare, D.K.; Caporini, M.A.; Bajaj, V.S.; Jaroniec, C.P.; Wang, L.; Ladizhansky, V.; Müller, S.A.; et al. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc. Natl. Acad. Sci. USA 2013, 110, 5468–5473. [Google Scholar] [CrossRef] [Green Version]
- Perutz, M.F. Amyloid fibrils. Mutations make enzyme polymerize. Nature 1997, 385, 773. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Vanacore, G.M.; Zewail, A.H. Nanomechanics and intermolecular forces of amyloid revealed by four-dimensional electron microscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 3380–3385. [Google Scholar] [CrossRef] [Green Version]
- Bucciantini, M.; Rigacci, S.; Stefani, M. Amyloid Aggregation: Role of Biological Membranes and the Aggregate–Membrane System. J. Phys. Chem. Lett. 2014, 5, 517–527. [Google Scholar] [CrossRef]
- Gillet, J.N. From molecular dynamics to quantum mechanics of misfolded proteins and amyloid-like macroaggregates applied to neurodegenerative diseases. J. Mol. Graph. Model. 2022, 112, 108046. [Google Scholar] [CrossRef]
- Come, J.H.; E Fraser, P.; Lansbury, P.T. A kinetic model for amyloid formation in the prion diseases: Importance of seeding. Proc. Natl. Acad. Sci. USA 1993, 90, 5959–5963. [Google Scholar] [CrossRef] [Green Version]
- Mocanu, M.-M.; Ganea, C.; Siposova, K.; Filippi, A.; Demjen, E.; Marek, J.; Bednarikova, Z.; Antosova, A.; Baran, I.; Gazova, Z. Polymorphism of hen egg white lysozyme amyloid fibrils influences the cytotoxicity in LLC-PK1 epithelial kidney cells. Int. J. Biol. Macromol. 2014, 65, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Beasley, K.L.; Weiss, R.A. Radiofrequency in Cosmetic Dermatology. Dermatol. Clin. 2014, 32, 79–90. [Google Scholar] [CrossRef]
- Suh, D.H.; Ahn, H.; Seo, J.; Lee, S.J.; Shin, M.K.; Song, K.Y. Monopolar radiofrequency treatment for facial laxity: Histometric analysis. J. Cosmet. Dermatol. 2020, 19, 2317–2324. [Google Scholar] [CrossRef]
- Sadick, N.S.; Nassar, A.H.; Dorizas, A.S.; Alexiades-Armenakas, M. Bipolar and Multipolar Radiofrequency. Dermatol. Surg. 2014, 40, S174–S179. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Intralesional methotrexate in dermatology: Diverse indications and practical considerations. Dermatol. Ther. 2020, 34, e14404. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrýsková, N.; Vrbovská, H.; Babincová, M.; Babinec, P.; Šimaljaková, M. Dissolution of Lysozyme Amyloid Fibrils Using Magnetic Nanoparticles in an Alternating Magnetic Field: Design of an Effective Treatment for Cutaneous Amyloidosis. Magnetochemistry 2023, 9, 84. https://doi.org/10.3390/magnetochemistry9030084
Andrýsková N, Vrbovská H, Babincová M, Babinec P, Šimaljaková M. Dissolution of Lysozyme Amyloid Fibrils Using Magnetic Nanoparticles in an Alternating Magnetic Field: Design of an Effective Treatment for Cutaneous Amyloidosis. Magnetochemistry. 2023; 9(3):84. https://doi.org/10.3390/magnetochemistry9030084
Chicago/Turabian StyleAndrýsková, Natália, Hana Vrbovská, Melánia Babincová, Peter Babinec, and Mária Šimaljaková. 2023. "Dissolution of Lysozyme Amyloid Fibrils Using Magnetic Nanoparticles in an Alternating Magnetic Field: Design of an Effective Treatment for Cutaneous Amyloidosis" Magnetochemistry 9, no. 3: 84. https://doi.org/10.3390/magnetochemistry9030084
APA StyleAndrýsková, N., Vrbovská, H., Babincová, M., Babinec, P., & Šimaljaková, M. (2023). Dissolution of Lysozyme Amyloid Fibrils Using Magnetic Nanoparticles in an Alternating Magnetic Field: Design of an Effective Treatment for Cutaneous Amyloidosis. Magnetochemistry, 9(3), 84. https://doi.org/10.3390/magnetochemistry9030084