Two One-Dimensional Copper-Oxalate Frameworks with the Jahn–Teller Effect: [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II)
Abstract
:1. Introduction
2. Experiment
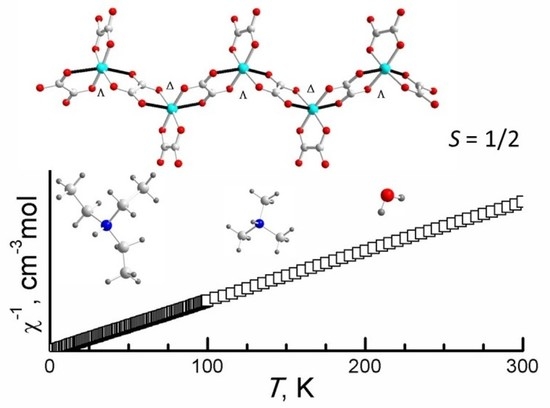
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states-I—Orbital degeneracy. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 161, 220–235. [Google Scholar]
- Opik, U.; Pryce, M.H.L. Studies of the Jahn-Teller effect. I. A survey of the static problem. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1957, 238, 425–427. [Google Scholar]
- Longuet-Higgins, H.C.; Opik, U.; Pryce, H.I.; Sack, R.A. Studies of the Jahn-Teller effect. II. The dynamical problem. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1958, 244, 1–16. [Google Scholar]
- Bednorz, J.G.; Muller, K.A. Possible high Tc superconductivity in the Ba−La−Cu−O system. Z. Für Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Radaelli, P.G.; Cox, D.E.; Marezio, M.; Cheong, S.W.; Schiffer, P.E.; Ramirez, A.P. Simultaneous Structural, Magnetic, and Electronic Transitions in La 1−x Cax MnO3 with x= 0.25 and 0.50. Phys. Rev. Lett. 1995, 75, 4488–4491. [Google Scholar] [CrossRef]
- Dagotto, E.; Hotta, T.; Moreo, A. Colossal magnetoresistant materials: The key role of phase separation. Phys. Rep. 2001, 344, 1–153. [Google Scholar] [CrossRef]
- Dagotto, E. Complexity in strongly correlated electronic systems. Science 2005, 309, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Jahn-Teller phenomena in solids. Annu. Rev. Mater. Sci. 1998, 28, 1–27. [Google Scholar] [CrossRef]
- Bersuker, I.B. The Jahn-Teller Effect; Cambridge University Press, Edinburgh Building: Cambridge, UK, 2006. [Google Scholar]
- Staub, U.; Scagnoli, V.; Muleders, A.M.; Janousch, M.; Honda, Z.; Tonnerre, J.M. Charge/orbital ordering vs. Jahn-Teller distortion in La0. 5Sr1. 5MnO4. Europhys. Lett. 2006, 76, 926–932. [Google Scholar] [CrossRef]
- Clement, R.; Decurtins, S.; Gruselle, M.; Train, C. Polyfunctional two-(2D) and three-(3D) dimensional oxalate bridged bimetallic magnets. Mon. Chem. 2003, 134, 117–135. [Google Scholar] [CrossRef]
- Daze, C.L.F.; Noa, M.A.; Nenwa, J.; Ohrstrom, L. Natural and synthetic metal oxalates–a topology approach. CrystEngComm 2019, 21, 6156–6164. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Light, M.E.; Price, D.J. One-Dimensional Magnetism in Anhydrous Iron and Cobalt Ternary Oxalates with Rare Trigonal-Prismatic Metal Coordination Environment. Angew. Chem. Int. Ed. 2004, 43, 472–475. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Chang, G.; Wang, Z.; Zhu, D. Crystal-to-Crystal Transformation from K2 [Co (C2O4)2(H2O)2]·4H2O to K2 [Co (μ-C2O4)(C2O4)]. Magnetochemistry 2021, 7, 77. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Co(C2O4)(HO(CH2)3OH): An antiferromagnetic neutral zigzag chain compound showing long-range ordering of spin canting. Inorg. Chem. 2008, 47, 9152–9154. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Fujiwara, H.; Kabayashi, H.; Kurmoo, M.; Inoue, K.; Mori, T.; Gao, S.; Zhang, Y.; Zhu, D. Tetrathiafulvalene [FeIII (C2O4)Cl2]: An Organic–Inorganic Hybrid Exhibiting Canted Antiferromagnetism. Adv. Mater. 2005, 17, 1988–1991. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Zhang, Y.; Takahashi, K.; Okano, Y.; Cui, H.; Kobayashi, H.; Inoue, K.; Kurmoo, M.; Pratt, F.; et al. Hybrid Organic—Inorganic Conductor with a Magnetic Chain Anion: κ-BETS2 [FeIII(C2O4)Cl2][BETS = Bis (ethylenedithio) tetraselenafulvalene]. Inorg. Chem. 2006, 45, 3275–3280. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Okawa, H. Design of metal-complex magnets. Syntheses and magnetic properties of mixed-metal assemblies {NBu4[MCr (ox)3]}x (NBu4+ = tetra (n-butyl) ammonium ion; ox2− = oxalate ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Mathoniere, C.; Nuttall, C.J.; Carling, S.G.; Day, P. Ferrimagnetic mixed-valency and mixed-metal tris (oxalato) iron (III) compounds: Synthesis, structure, and magnetism. Inorg. Chem. 1996, 35, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Two Homometallic Antiferromagnets Based on Oxalato-Bridged Honeycomb Assemblies:(A)2[MII(C2O4)3](A= Ammonium Salt Derived from Diethylenetriamine; MII= Fe2+, Co2+). Inorg. Chem. 2009, 48, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Rousse, G.; Rodriguez-Carvajal, J. Oxalate-mediated long-range antiferromagnetism order in Fe2(C2O4)3 4H2O. Dalton Trans. 2016, 45, 14311–14316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhang, J.; Li, J.; Zhu, D. A neutral molecular-based layered magnet [Fe (C2O4)(CH3OH)]n exhibiting magnetic ordering at TN≈ 23 K. Dalton Trans. 2008, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Esling, J.; Gutlich, P. A concept for the synthesis of 3-dimensional homo-and bimetallic oxalate-bridged networks [M2(ox)3]n. Structural, moessbauer, and magnetic studies in the field of molecular-based magnets. J. Am. Chem. Soc. 1994, 116, 9521–9528. [Google Scholar] [CrossRef]
- Hernandez-Molina, M.; Lloret, F.; Ruiz-Perez, C.; Julve, M. Weak Ferromagnetism in chiral 3-dimensional oxalato-bridged cobalt (II) compounds. Crystal structure of [Co(bpy)3][Co2(ox)3]ClO4. Inorg. Chem. 1998, 37, 4131–4135. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garzia, C.J.; Martinez-Agudo, J.M. Molecule-based magnets formed by bimetallic three-dimensional oxalate networks and chiral tris (bipyridyl) complex cations. The series [ZII (bpy)3][ClO4][MIICrIII (ox)3](ZII= Ru, Fe, Co, and Ni; MII= Mn, Fe, Co, Ni, Cu, and Zn; ox= oxalate dianion). Inorg. Chem. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhang, J.; Hao, X.; Zhu, D. Mn (C2O4)(H2O)0.25: An antiferromagnetic oxalato-based cage compound. Dalton Trans. 2011, 40, 5430–5432. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Grancha, T.; Verdaguer, M.; Train, C.; Armentano, D.; Pardo, E. Solvent-Dependent Self-Assembly of an Oxalato-Based Three-Dimensional Magnet Exhibiting a Novel Architecture. Inorg. Chem. 2016, 55, 6845–6847. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Marti-Gastaldo, C. Single chain magnets based on the oxalate ligand. J. Am. Chem. Soc. 2008, 130, 14987–14989. [Google Scholar] [CrossRef]
- Anderson, P.W. Resonating valence bonds: A new kind of insulator? Mater. Res. Bull. 1973, 8, 153–160. [Google Scholar] [CrossRef]
- Anderson, P.W. The resonating valence bond state in La2CuO4 and superconductivity. Science 1987, 235, 1196–1198. [Google Scholar] [CrossRef]
- Ramirez, A.P. Strongly geometrically frustrated magnets. Annu. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
- Balent, I. Spin liquids in frustrated magnets. Nature 2010, 464, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3Cu2(C2O4)3(CH3OH)2: An organic–inorganic hybrid antiferromagnetic semiconductor. Chem. Commun. 2012, 48, 197–198. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liu, F.; Zhu, D. BETS3[Cu2(C2O4)3](CH3OH)2: An organic–inorganic hybrid antiferromagnetic metal (BETS = bisethylene(tetraselenfulvalene)). CrystEngComm 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Wang, D.; Yang, D.; Gao, Z.; Chang, G.; Guo, Y.; Mori, T.; Zhao, Z.; et al. Organic–inorganic hybrid metallic conductors based on bis (ethylenedithio) tetrathiafulvalene cations and antiferromagnetic oxalate-bridged copper (ii) dinuclear anions. J. Mater. Chem. C 2022, 10, 2845–2852. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Wang, D.; Baker, P.; Pratt, F.; Zhu, D. Candidate quantum spin liquid due to dimensional reduction of a two-dimensional honeycomb lattice. Sci. Rep. 2014, 4, 6451. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhu, D. [(C2H5)3NH]2Cu2(C2O4)3: A three-dimensional metal–oxalato framework showing structurally related dielectric and magnetic transitions at around 165 K. Dalton Trans. 2012, 14, 8509–8511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Baker, P.; Zhang, Y.; Wang, D.; Wang, Z.; Su, S.; Zhu, D.; Pratt, F.L. Quantum spin liquid from a three-dimensional copper-oxalate framework. J. Am. Chem. Soc. 2018, 140, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Shelx-97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Bain, A.B.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Zhang, B. CCDC843075. Available online: http://www.ccdc.cam.ac.uk (accessed on 5 September 2011).
- Vilela, R.S.; Oliveira, T.I.; Martins, F.T.; Ellena, J.A.; Lloret, F.; Julve, M.; Cangussu, D. Synthesis, crystal structure and magnetic properties of the helical oxalate-bridged copper(II) chain {[(CH3)4N]2[Cu(C2O4)2]·H2O}n. Comptes Rendus Chim. 2012, 15, 856–865. [Google Scholar] [CrossRef]
- Cano, J.; Alemany, P.; Alvarez, M.; Verdaguer, M.; Ruiz, E. Exchange Coupling in Oxalato-Bridged Copper (ii) Binuclear Compounds: A Density Functional Study. Chem. A Eur. J. 1998, 4, 476–484. [Google Scholar] [CrossRef]
- Carlin, R.; van Duyneveldt, A. Magnetic Properties of Transition Metal Compounds; Springer: New York, NY, USA, 1977; p. 69. [Google Scholar]
- Zhang, B.; Zhang, Y.; Zhang, J.; Yan, X.; Zhu, D. Step by step crystal-to-crystal transformation from 1D K2Cu(C2O4)2(H2O)4 (1) to 1D K2Cu(C2O4)2(H2O)2 (2) and then 1D K2Cu(C2O4)2 (3) by dehydration. CrystEngComm 2016, 18, 5062–5065. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publisher Inc.: New York, NY, USA, 1993; pp. 10–29. [Google Scholar]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A beginner’s guide to interpreting magnetic susceptibility data with the Curie-Weiss law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Baker, G.A.; Rushbrooke, G.S.; Gilbert, H.E. High-temperature series expansions for the spin-½ Heisenberg model by the method of irreducible representations of the symmetric group. Phys. Rev. 1964, 135, A1272–A1277. [Google Scholar] [CrossRef]
- Carling, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; pp. 132–133. [Google Scholar]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Gracia, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Alberola, A.; Coronado, E.; Galan-Mascaros, J.R.; Gimenez-Saiz, C.; Gomez-Garcia, C.J. A molecular metal ferromagnet from the organic donor bis (ethylenedithio) tetraselenafulvalene and bimetallic oxalate complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, Y.; Wang, Z.; Sun, Y.; Liang, T.; Liu, M.; Zhu, D. Two One-Dimensional Copper-Oxalate Frameworks with the Jahn–Teller Effect: [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II). Magnetochemistry 2023, 9, 120. https://doi.org/10.3390/magnetochemistry9050120
Zhang B, Zhang Y, Wang Z, Sun Y, Liang T, Liu M, Zhu D. Two One-Dimensional Copper-Oxalate Frameworks with the Jahn–Teller Effect: [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II). Magnetochemistry. 2023; 9(5):120. https://doi.org/10.3390/magnetochemistry9050120
Chicago/Turabian StyleZhang, Bin, Yan Zhang, Zheming Wang, Yang Sun, Tongling Liang, Mei Liu, and Daoben Zhu. 2023. "Two One-Dimensional Copper-Oxalate Frameworks with the Jahn–Teller Effect: [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II)" Magnetochemistry 9, no. 5: 120. https://doi.org/10.3390/magnetochemistry9050120
APA StyleZhang, B., Zhang, Y., Wang, Z., Sun, Y., Liang, T., Liu, M., & Zhu, D. (2023). Two One-Dimensional Copper-Oxalate Frameworks with the Jahn–Teller Effect: [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II). Magnetochemistry, 9(5), 120. https://doi.org/10.3390/magnetochemistry9050120