Nontrivial Effects of “Trivial” Parameters on the Performance of Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
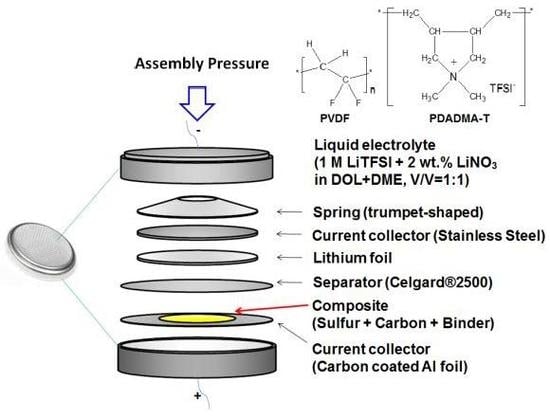
2.2. Electrode Fabrication
2.3. Electrochemical Testing
3. Results and Discussion
3.1. Effect of Spring Thickness
3.2. Effect of Assembling Pressure and Li-Foil Thickness
3.3. Effect of Sheet Number of Celgard 2500 Separator
3.4. Effect of Cut-Off Voltage
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Manthiram, A.; Chung, S.H.; Zu, C. Lithium–sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Urbonaite, S.; Poux, T.; Novák, P. Progress towards commercially viable Li–S battery cells. Adv. Energy Mater. 2015, 5, 1500118. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li–sulfur battery systems: An integral perspective. Adv. Energy Mater. 2015, 5, 150–212. [Google Scholar] [CrossRef]
- Bruckner, J.; Thieme, S.; Grossmann, H.T.; Dorfler, S.; Althues, H.; Kaskel, S. Lithium–sulfur batteries: Influence of C-rate, amount of electrolyte and sulfur loading on cycle performance. J. Power Sources 2014, 268, 82–87. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Rauh, R.D.; Abraham, K.M.; Pearson, G.F.; Surprenant, J.K.; Brummer, S.B. A lithium/dissolved sulfur battery with an organic electrolyte. J. Electrochem. Soc. 1979, 126, A523–A527. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.R.; Adelhelm, P.; Sommer, H.; Schneider, H.; Leitner, K.; Janek, J. Systematical electrochemical study on the parasitic shuttle-effect in lithium-sulfur-cells at different temperatures and different rates. J. Power Sources 2014, 259, 289–299. [Google Scholar] [CrossRef]
- Urbonaite, S.; Novak, P. Importance of “unimportant” experimental parameters in Li-S battery development. J. Power Sources 2014, 249, 497–502. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. A membrane-free lithium-polysulfide semi-liquid battery for large-scale energy storage. Energy Environ. Sci. 2013, 6, 1552–1558. [Google Scholar] [CrossRef]
- Knap, V.; Stroe, D.I.; Swierczynski, M.; Teodorescu, R.; Schaltz, E. Investigation of the self-discharge behavior of lithium-sulfur batteries. J. Electrochem. Soc. 2016, 163, A911–A916. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Qin, X.; Liang, G.; Han, W.; Zhou, D.; He, Y.B.; Li, B.; Kang, F. Suppressing self-discharge and shuttle effect of lithium-sulfur batteries with V2O5-decorated carbon nanofiber interlayer. Small 2017, 13, 1602539. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Huang, J.Q.; Peng, H.J.; Nie, J.Q.; Liu, X.Y.; Zhang, Q.; Wei, F. Polysulfide shuttle control: Towards a lithium-sulfur battery with superior capacity performance up to 1000 cycles by matching the sulfur/electrolyte loading. J. Power Sources 2014, 253, 263–268. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Li, H.; Ke, H.; Zeng, D.; Zhang, Y.; Sun, Y.; Cheng, H. Explore the influence of coverage percentage of sulfur electrode on the cycle performance of lithium-sulfur batteries. J. Power Sources 2017, 347, 238–246. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Yuan, L.; Hao, Z.; Huang, Y. Status and prospects in sulfur-carbon composites as cathode materials for rechargeable lithium-sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in Lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Khan, K.; Zhao, Y.; Hou, Y. Nanostructured cathode materials for lithium-sulfur batteries: Progress, challenges and perspectives. J. Mater. Chem. A 2017, 5, 3014–3038. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Y.; Zhao, X.; Wang, X.; Du, G.; Zhang, J.; Tu, J. Sulfur/three-dimensional graphene composite for high performance lithium-sulfur batteries. J. Power Sources 2015, 275, 22–25. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Ding, Y.; Zhang, W.; Yu, G. In situ reactive synthesis of polypyrrole-MnO2 coaxial nanotubes as sulfur hosts for high-performance lithium-sulfur battery. Nano Lett. 2016, 16, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y.; Ding, Y.; Peng, L.; Zhang, W.; Yu, G. A conductive molecular framework derived Li2S/N,P-codoped carbon cathode for advanced lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1602876. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.H.; Zhang, W.; Manthiram, A.; Yu, G. Nanostructured host materials for trapping sulfur in rechargeable Li-S batteries: Structure design and interfacial chemistry. Small Methods 2017, 1700279. [Google Scholar] [CrossRef]
- Lim, S.; Thankamony, R.L.; Yim, T.; Chu, H.; Kim, Y.J.; Mun, J.; Kim, T.Y. Surface Modification of sulfur electrodes by chemically anchored cross-linked polymer coating for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2015, 7, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, L.; Li, Z.; Qi, Y.; Wu, C.; Liu, J.; Huang, Y. Improving the electrochemical performance of a lithium-sulfur battery with a conductive polymer coated sulfur cathode. RSC Adv. 2015, 5, 44160–44164. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, T.; Lee, Y.; Lee, J.K.; Choi, W. Coating of sulfur particles with manganese oxide nanowires as a cathode material in lithium–sulfur batteries. Mater. Lett. 2015, 158, 132–135. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries: Progress and prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Xu, G.; Yan, Q.B.; Kushima, A.; Zhang, X.; Pan, J.; Li, J. Conductive graphene oxide-polyacrylic acid (GOPAA) binder for lithium–sulfur battery. Nano Energy 2017, 31, 568–574. [Google Scholar] [CrossRef]
- Chen, W.; Qian, T.; Xiong, J.; Xu, N.; Liu, X.J.; Liu, J.; Zhou, J.; Shen, X.; Yang, T.; Chen, Y.; et al. A new type of multifunctional polar binder: Toward practical application of high energy lithium sulfur batteries. Adv. Mater. 2017, 29, 1605160. [Google Scholar] [CrossRef] [PubMed]
- Milroy, C.; Manthiram, A. An Elastic, conductive, electroactive nanocomposite binder for flexible sulfur cathodes in lithium-sulfur batteries. Adv. Mater. 2016, 28, 9744. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the surface chemical aspects of very high energy density, rechargeable Li–sulfur batteries. J. Electrochem. Soc. 2009, 156, A694–A702. [Google Scholar] [CrossRef]
- Zhang, S.S. A new finding on the role of LiNO3 in lithium-sulfur battery. J. Power Sources 2016, 322, 99–105. [Google Scholar] [CrossRef]
- Barchasz, C.; Leprêtre, J.C.; Alloin, F.; Patoux, S. New insights into the limiting parameters of the Li–S rechargeable cell. J. Power Sources 2012, 199, 322–330. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Kiya, Y.; Abrunña, H.D. Effects of liquid electrolytes on the charge discharge performance of rechargeable lithium/sulfur batteries: Electrochemical and in-situ X-ray absorption spectroscopic studies. J. Phys. Chem. C 2011, 115, 25132–25137. [Google Scholar] [CrossRef]
- Zhang, S.S. Improved cyclability of liquid electrolyte lithium/sulfur batteries by optimizing electrolyte/sulfur ratio. Energies 2012, 5, 5190–5197. [Google Scholar] [CrossRef]
- Zhang, S.S. Effect of discharge cutoff voltage on reversibility of lithium/sulfur batteries with LiNO3-contained electrolyte. J. Electrochem. Soc. 2012, 159, A920–A923. [Google Scholar] [CrossRef]
- Ding, N.; Chien, S.W.; Andy Hor, T.S.; Liu, Z.; Zong, Y. Key parameters in design of lithium sulfur batteries. J. Power Sources 2014, 269, 111–116. [Google Scholar] [CrossRef]
- Gao, J.; Abrunña, H.D. Key parameters governing the energy density of rechargeable Li/S batteries. J. Phys. Chem. Lett. 2014, 5, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Ye, Z. Quaternary ammonium cationic polymer as a superior bifunctional binder for lithium-sulfur batteries and effects of counter anion. Electrochim. Acta 2017, 259, 626–636. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Hart, C.J.; Cuisinier, M.; Liang, X.; Kundu, D.; Garsuch, A.; Nazar, L.F. Rational design of sulphur host materials for Li-S Batteries: Correlating lithium polysulphide adsorptivity and self-discharge capacity loss. Chem. Commun. 2015, 51, 2308–2311. [Google Scholar] [CrossRef] [PubMed]
- Moy, D.; Manivannan, A.; Narayanana, S.R. Direct measurement of polysulfide shuttle current: A window into understanding the performance of lithium-sulfur cells. J. Electrochem. Soc. 2015, 162, A1–A7. [Google Scholar] [CrossRef]
- Wu, F.; Kim, H.; Magasinski, A.; Lee, J.T.; Lin, H.T.; Yushin, G. Harnessing steric separation of freshly nucleated Li2S nanoparticles for bottom-up assembly of high-performance cathodes for lithium-sulfur and lithium-ion batteries. Adv. Energy Mater. 2014, 4, 140–196. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, N.; Wang, M.; Qu, C.; Yang, X.; Zhang, H.; Li, X.; Zhang, H. Layer-by-layer assembled C/S cathode with trace binder for Li-S battery application. ACS Appl. Mater. Interfaces 2015, 7, 25002–25006. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Shan, Z.; Wang, L.; Qin, X.; Zhu, K.; Tian, J.; Liu, X. Nafion coated sulfur-carbon electrode for high performance lithium-sulfur batteries. J. Power Sources 2014, 246, 253–259. [Google Scholar] [CrossRef]
- Pan, J.; Xu, G.; Ding, B.; Chang, Z.; Wang, A.; Dou, H.; Zhang, X. PAA/PEDOT: PSS as a multifunctional, water soluble binder to improve the capacity and stability of lithium–sulfur batteries. RSC Adv. 2016, 6, 40650–40655. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in lithium ion batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yanilmaz, M.; Fu, K.; Chen, C.; Lu, Y.; Ge, Y.; Kim, D.; Zhang, X. Understanding glass fiber membrane used as a novel separator for lithium-sulfur batteries. J. Membr. Sci. 2016, 504, 89–96. [Google Scholar] [CrossRef]
- He, G.; Hart, C.J.; Liang, X.; Garsuch, A.; Nazar, L.F. Stable cycling of a scalable graphene-encapsulated nanocomposite for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2014, 6, 10917–10923. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.S.; Fu, Y.; Manthiram, A. Self-weaving sulfur-carbon composite cathodes for high rate lithium-sulfur batteries. Phys. Chem. Chem. Phys. 2012, 14, 14495–14499. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Jin, J.; Wen, Z.; Zhang, S.; Wang, Q.; Shen, C.; Rui, K. On the dispersion of lithium-sulfur battery cathode materials effected by electrostatic and stereo-chemical factors of binders. J. Power Sources 2016, 324, 455–461. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Ye, Z. Nontrivial Effects of “Trivial” Parameters on the Performance of Lithium–Sulfur Batteries. Batteries 2018, 4, 22. https://doi.org/10.3390/batteries4020022
Liao J, Ye Z. Nontrivial Effects of “Trivial” Parameters on the Performance of Lithium–Sulfur Batteries. Batteries. 2018; 4(2):22. https://doi.org/10.3390/batteries4020022
Chicago/Turabian StyleLiao, Junbin, and Zhibin Ye. 2018. "Nontrivial Effects of “Trivial” Parameters on the Performance of Lithium–Sulfur Batteries" Batteries 4, no. 2: 22. https://doi.org/10.3390/batteries4020022
APA StyleLiao, J., & Ye, Z. (2018). Nontrivial Effects of “Trivial” Parameters on the Performance of Lithium–Sulfur Batteries. Batteries, 4(2), 22. https://doi.org/10.3390/batteries4020022