Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc–Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the CMC-CS and CMC-CS-CA Hydrogels
2.2. Swelling Behavior of the Hydrogels
2.3. Structural, Thermal, and Electrochemical Characterization
2.3.1. ATR-FTIR Methods
2.3.2. XRD Characterization
2.3.3. SEM and BET Characterization
2.3.4. Thermal Analysis
2.3.5. Electrochemical Measurements
2.4. Battery Tests
3. Results
3.1. Formation Reaction of the Hydrogels
3.2. Swelling Behavior
3.3. Structural Characterization
3.3.1. ATR-FTIR Analysis
3.3.2. XRD Analysis
3.3.3. SEM Micrographs and BET Analysis
3.4. Thermal Characterization
3.5. Electrochemical Characterization
3.5.1. Influence of the CA Content on the Ionic Conductivity
3.5.2. Linear Sweep and Cyclic Voltammetry (CV)
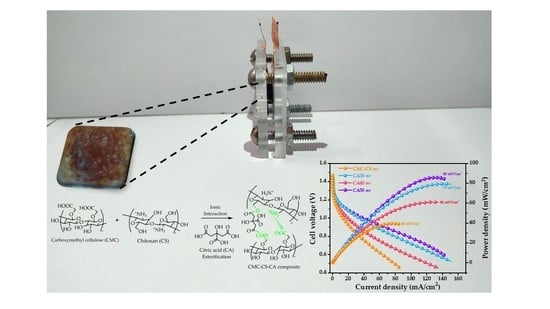
3.6. Zn/Hydrogel/Air Battery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Chen, Z.; Fang, G.; Wang, Z.; Cai, Y.; Tang, B.; Zhou, J.; Liang, S. V2O5 Nanospheres with Mixed Vanadium Valences as High Electrochemically Active Aqueous Zinc-Ion Battery Cathode. Nano-Micro Lett. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Deivanayagam, R.; Ingram, B.J.; Shahbazian-Yassar, R. Progress in development of electrolytes for magnesium batteries. Energy Storage Mater. 2019, 21, 136–153. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Boaretto, N.; Meabe, L.; Martinez-Ibañez, M.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Rechargeable Batteries: From Nanocomposite to Nanohybrid. J. Electrochem. Soc. 2020, 167, 070524. [Google Scholar] [CrossRef]
- Fang, G.; Zhu, C.; Chen, M.; Zhou, J.; Tang, B.; Cao, X.; Zheng, X.; Pan, A.; Liang, S. Suppressing Manganese Dissolution in Potassium Manganate with Rich Oxygen Defects Engaged High-Energy-Density and Durable Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2019, 29, 1808375. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Z.; Wu, X.; Wen, Y.; Chen, H.; Ni, X.; Liu, G.; Huang, J.; Peng, S. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries. J. Energy Chem. 2022, 64, 23–32. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Guo, H.; He, S.; Yang, H.; Liu, K.; Duan, G.; Jiang, S. Advanced electrospun nanofibers as bifunctional electrocatalysts for flexible metal-air (O2) batteries: Opportunities and challenges. Mater. Des. 2022, 214, 110406. [Google Scholar] [CrossRef]
- Li, D.; Lv, Q.; Zhang, C.; Zhou, W.; Guo, H.; Jiang, S.; Li, Z. The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery. Batteries 2022, 8, 101. [Google Scholar] [CrossRef]
- Zaghib, K.; Song, S.-W.; Singh, K.; Yao, Y.; Ichikawa, T.; Jain, A.; Singh, R. Zinc as a Promising Anodic Material for All-Solid-State Lithium-Ion Batteries. Batteries 2022, 8, 113. [Google Scholar] [CrossRef]
- Lu, C.-T.; Zhu, Z.-Y.; Chen, S.-W.; Chang, Y.-L.; Hsueh, K.-L. Effects of Cell Design Parameters on Zinc-Air Battery Performance. Batteries 2022, 8, 92. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.Z.; Ansari, M.O. Manganese oxide as an effective electrode material for energy storage: A review. Environ. Chem. Lett. 2021, 20, 283–309. [Google Scholar] [CrossRef]
- He, W.; Zuo, S.; Xu, X.; Zeng, L.; Liu, L.; Zhao, W.; Liu, J. Challenges and strategies of zinc anode for aqueous zinc-ion batteries. Mater. Chem. Front. 2021, 5, 2201–2217. [Google Scholar] [CrossRef]
- Ye, T.; Li, L.; Zhang, Y. Recent Progress in Solid Electrolytes for Energy Storage Devices. Adv. Funct. Mater. 2020, 30, 2000077. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.-S.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, M. Toward Dendrite-Free Deposition in Zinc-Based Flow Batteries: Status and Prospects. Batteries 2022, 8, 117. [Google Scholar] [CrossRef]
- Wood, K.N.; Kazyak, E.; Chadwick, A.F.; Chen, K.-H.; Zhang, J.-G.; Thornton, K.; Dasgupta, N.P. Dendrites and Pits: Untangling the Complex Behavior of Lithium Metal Anodes through Operando Video Microscopy. ACS Cent. Sci. 2016, 2, 790–801. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.-X.; Cheng, X.-B.; Bai, Y.; Li, Y.; Wu, C.; Zhang, Q. Perspectives for restraining harsh lithium dendrite growth: Towards robust lithium metal anodes. Energy Storage Mater. 2018, 15, 148–170. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, D.T.; Villaluenga, I.; Balsara, N.P. Polymer and composite electrolytes. MRS Bull. 2018, 43, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Bósquez-Cáceres, M.F.; Hidalgo-Bonilla, S.; Córdova, V.M.; Michell, R.M.; Tafur, J.P. Nanocomposite Polymer Electrolytes for Zinc and Magnesium Batteries: From Synthetic to Biopolymers. Polymers 2021, 13, 4284. [Google Scholar] [CrossRef]
- Mo, F.; Guo, B.; Ling, W.; Wei, J.; Chen, L.; Yu, S.; Liang, G. Recent Progress and Challenges of Flexible Zn-Based Batteries with Polymer Electrolyte. Batteries 2022, 8, 59. [Google Scholar] [CrossRef]
- Wu, K.; Huang, J.; Yi, J.; Liu, X.; Liu, Y.; Wang, Y.; Zhang, J.; Xia, Y. Recent Advances in Polymer Electrolytes for Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. Adv. Energy Mater. 2020, 10, 209–216. [Google Scholar] [CrossRef]
- Lorca, S.; Santos, F.; Fernández Romero, A.J. A Review of the Use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles. Polymers 2020, 12, 2812. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Subban, R.H.Y.; Arof, A.K. Polymer batteries fabricated from lithium complexed acetylated chitosan. J. Power Sources 1995, 56, 153–156. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Rosca, C.; Popa, M.I.; Lisa, G.; Chitanu, G.C. Interaction of chitosan with natural or synthetic anionic polyelectrolytes. 1. The chitosan–carboxymethylcellulose complex. Carbohydr. Polym. 2005, 62, 35–41. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Electrical Behavior of a Natural Polyelectrolyte Hydrogel: Chitosan/Carboxymethylcellulose Hydrogel. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Bakar, N.Y.; Isa, M.I.N. Potential of Ionic Conductivity and Transport Properties Solid Biopolymer Electrolytes Based Carboxy Methylcellulose/Chitosan Polymer Blend Doped with Dodecyltrimethyl Ammonium Bromide. Res. J. Recent Sci. 2014, 3, 74. [Google Scholar]
- Rani, M.S.A.; Mohamed, N.S.; Isa, M.I.N. Investigation of the Ionic Conduction Mechanism in Carboxymethyl Cellulose/Chitosan Biopolymer Blend Electrolyte Impregnated with Ammonium Nitrate. Int. J. Polym. Anal. Charact. 2015, 20, 491–503. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C.; Wang, Z.F.; et al. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Liu, J.; Ahmed, S.; Khanam, Z.; Wang, T.; Song, S. Ionic Liquid-Incorporated Zn-Ion Conducting Polymer Electrolyte Membranes. Polymers 2020, 12, 1755. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Removal of Pb(ii) and Cd(ii) from wastewater using arginine cross-linked chitosan–carboxymethyl cellulose beads as green adsorbent. RSC Adv. 2019, 9, 7890–7902. [Google Scholar] [CrossRef] [Green Version]
- Salama, H.E.; Abdel Aziz, M.S.; Alsehli, M. Carboxymethyl cellulose/sodium alginate/chitosan biguanidine hydrochloride ternary system for edible coatings. Int. J. Biol. Macromol. 2019, 139, 614–620. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Daoud, W.A. Green and sustainable carboxymethyl cellulose-chitosan composite hydrogels: Effect of crosslinker on microstructure. Cellulose 2021, 28, 5493–5512. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Daoud, W.A. Carboxymethyl cellulose-chitosan composite hydrogel: Modelling and experimental study of the effect of composition on microstructure and swelling response. Int. J. Biol. Macromol. 2021, 181, 1010–1022. [Google Scholar] [CrossRef]
- Santos, F.; Tafur, J.P.; Abad, J.; Fernández Romero, A.J. Structural modifications and ionic transport of PVA-KOH hydrogels applied in Zn/Air batteries. J. Electroanal. Chem. 2019, 850, 113380. [Google Scholar] [CrossRef]
- Lewandowski, A.; Skorupska, K.; Malinska, J. Novel poly(vinyl alcohol)–KOH–H2O alkaline polymer electrolyte. Solid State Ion. 2000, 133, 265–271. [Google Scholar] [CrossRef]
- Velez, A.A.I.; Reyes, E.; Diaz-Barrios, A.; Santos, F.; Fernández Romero, A.J.; Tafur, J.P. Properties of the PVA-VAVTD KOH Blend as a Gel Polymer Electrolyte for Zinc Batteries. Gels 2021, 7, 256. [Google Scholar] [CrossRef]
- Calderón Salas, L.A.; De Lima Eljuri, L.; Caetano Sousa, M. Synthesis and Characterization of Chemically Crosslinked Carboxymethyl Cellulose/Chitosan Composite Hydrogels; Universidad de Investigación de Tecnología Experimental Yachay: Urcuqui, Ecuador, 2021. [Google Scholar]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction. Available online: https://www.crystalimpact.de/match (accessed on 16 August 2022).
- Nuernberg, R.B. Numerical comparison of usual Arrhenius-type equations for modeling ionic transport in solids. Ionics 2020, 26, 2405–2412. [Google Scholar] [CrossRef]
- Chandra Roy, J.; Ferri, A.; Giraud, S.; Jinping, G.; Salaün, F. Chitosan–Carboxymethylcellulose-Based Polyelectrolyte Complexation and Microcapsule Shell Formulation. Int. J. Mol. Sci. 2018, 19, 2521. [Google Scholar] [CrossRef] [Green Version]
- Seki, Y.; Altinisik, A.; Demircioğlu, B.; Tetik, C. Carboxymethylcellulose (CMC)–hydroxyethylcellulose (HEC) based hydrogels: Synthesis and characterization. Cellulose 2014, 21, 1689–1698. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of carboxymethylcellulose/acrylic acid hydrogels with superabsorbent properties by radiation-initiated crosslinking. Radiat. Phys. Chem. 2016, 124, 135–139. [Google Scholar] [CrossRef]
- Bajpai, J.; Mishra, S.; Bajpai, A.K. Dynamics of controlled release of potassium nitrate from a highly swelling binary polymeric blend of alginate and carboxymethyl cellulose. J. Appl. Polym. Sci. 2007, 106, 961–972. [Google Scholar] [CrossRef]
- Johns, J.; Rao, V. Mechanical Properties and Swelling Behavior of Cross-Linked Natural Rubber/Chitosan Blends. Int. J. Polym. Anal. Charact. 2009, 14, 508–526. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Pei, P.; Zuo, Y.; Wei, M.; Liu, X.; Xiao, Y.; Xiong, J. Selection of hydrogel electrolytes for flexible zinc–air batteries. Mater. Today Chem. 2021, 21, 100538. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Kadir, M.F.Z.; Aspanut, Z.; Majid, S.R.; Arof, A.K. FTIR studies of plasticized poly(vinyl alcohol)–chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- He, X.; Xu, H.; Li, H. Cr(VI) Removal from Aqueous Solution by Chitosan/Carboxylmethyl Cellulose/Silica Hybrid Membrane. World J. Eng. Technol. 2015, 3, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Biswal, D.R.; Singh, R.P. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Kuan, E.C.H.; Isa, M.I.N. Investigation of the Potential of Proton-Conducting Biopolymer Electrolytes Based Methyl Cellulose-Glycolic Acid. Int. J. Polym. Anal. Charact. 2011, 16, 477–485. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Mohamed, N.S.; Isa, M.I.N. Characterization of Proton Conducting Carboxymethyl Cellulose/Chitosan Dual-Blend Based Biopolymer Electrolytes. Mater. Sci. Forum 2016, 846, 539–544. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Kittur, F.S.; Tharanathan, R.N. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohydr. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.-Y.; Yu, C.-C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef]
- Farinha, I.; Freitas, F. Chemically modified chitin, chitosan, and chitinous polymers as biomaterials. Handb. Chitin Chitosan 2020, 43–69. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Son, Y.-R.; Rhee, K.Y.; Park, S.-J. Influence of reduced graphene oxide on mechanical behaviors of sodium carboxymethyl cellulose. Compos. Part B Eng. 2015, 83, 36–42. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Li, C.; Zhang, D.; Xiao, Y.; Guan, G.; Zhu, W. Modification of chitosan with monomethyl fumaric acid in an ionic liquid solution. Carbohydr. Polym. 2015, 117, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Abidin, Z.H.Z. Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 2015, 132, 41774. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, L.; Zhang, R.; Liu, G.; Cheng, G. Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Bioresour. Technol. 2014, 151, 402–405. [Google Scholar] [CrossRef]
- Abdullah, O.G.H.; Hanna, R.R.; Salman, Y.A.K. Structural and electrical conductivity of CH:MC bio-poly-blend films: Optimize the perfect composition of the blend system. Bull. Mater. Sci. 2019, 42, 64. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.A.M.; Gh Abdullah, O. Membranes. Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes 2020, 10, 262. [Google Scholar] [CrossRef]
- Sing Ngai, K.; Ramesh, S.; Ramesh, K.; Ching Juan, J. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, X.; Daoud, W.A. High power-output mechanical energy harvester based on flexible and transparent Au nanoparticle-embedded polymer matrix. Nano Energy 2019, 55, 433–440. [Google Scholar] [CrossRef]
- Ritonga, H.; Nurfadillah, A.; Rembon, F.S.; Ramadhan, L.O.A.N.; Nurdin, M. Preparation of Chitosan-EDTA hydrogel as soil conditioner for soybean plant (Glycine max). Groundw. Sustain. Dev. 2019, 9, 100277. [Google Scholar] [CrossRef]
- Suppiah, K.; Leng, T.P.; Husseinsyah, S.; Rahman, R.; Keat, Y.C.; Heng, C.W. Thermal properties of carboxymethyl cellulose (CMC) filled halloysite nanotube (HNT) bio-nanocomposite films. Mater. Today Proc. 2019, 16, 1611–1616. [Google Scholar] [CrossRef]
- Rana, V.K.; Pandey, A.K.; Singh, R.P.; Kumar, B.; Mishra, S.; Ha, C.-S. Enhancement of thermal stability and phase relaxation behavior of chitosan dissolved in aqueous l-lactic acid: Using ‘silver nanoparticles’ as nano filler. Macromol. Res. 2010, 18, 713–720. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Broström, M. Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Strydom, C.A.; Collins, A.C.; Bunt, J.R. The influence of various potassium compound additions on the plasticity of a high-swelling South African coal under pyrolyzing conditions. J. Anal. Appl. Pyrolysis 2015, 112, 221–229. [Google Scholar] [CrossRef]
- Wang, K.; Du, L.; Zhang, C.; Lu, Z.; Lu, F.; Zhao, H. Preparation of chitosan/curdlan/carboxymethyl cellulose blended film and its characterization. J. Food Sci. Technol. 2019, 56, 5396–5404. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M. Antimicrobial finish of textiles by chitosan UV-curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef]
- Hatta, F.F.; Yahya, M.Z.A.; Ali, A.M.M.; Subban, R.H.Y.; Harun, M.K.; Mohamad, A.A. Electrical conductivity studies on PVA/PVP-KOH alkaline solid polymer blend electrolyte. Ionics 2005, 11, 418–422. [Google Scholar] [CrossRef]
- Petrowsky, M.; Frech, R. Application of the Compensated Arrhenius Formalism to Self-Diffusion: Implications for Ionic Conductivity and Dielectric Relaxation. J. Phys. Chem. B 2011, 114, 8600–8605. [Google Scholar] [CrossRef]
- Pandey, G.P.; Agrawal, R.C.; Hashmi, S.A. Magnesium ion-conducting gel polymer electrolytes dispersed with fumed silica for rechargeable magnesium battery application. J. Solid State Electrochem. 2010, 15, 2253–2264. [Google Scholar] [CrossRef]
- Maheshwaran, C.; Mishra, K.; Kanchan, D.K.; Kumar, D. Mg2+ conducting polymer gel electrolytes: Physical and electrochemical investigations. Ionics 2020, 26, 2969–2980. [Google Scholar] [CrossRef]
- Rahman, N.A.; Hanifah, S.A.; Mobarak, N.N.; Ahmad, A.; Ludin, N.A.; Bella, F.; Su’Ait, M.S. Chitosan as a paradigm for biopolymer electrolytes in solid-state dye-sensitised solar cells. Polymer 2021, 230, 124092. [Google Scholar] [CrossRef]
- Dannoun, E.M.A.; Aziz, S.B.; Brza, M.A.; Nofal, M.M.; Asnawi, A.S.F.M.; Yusof, Y.M.; Al-Zangana, S.; Hamsan, M.H.; Kadir, M.F.Z.; Woo, H.J. The Study of Plasticized Solid Polymer Blend Electrolytes Based on Natural Polymers and Their Application for Energy Storage EDLC Devices. Polymers 2020, 12, 2531. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Ahmed, S.; Wazed Ali, S.; Agarwal, H. Chitosan-based polymer electrolyte membranes for fuel cell applications. Org. Compos. Polym. Electrolyte Membr. Prep. Prop. Fuel Cell Appl. 2017, 381–398. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.; Liu, W.; Liu, H.; Dai, L.; Zhang, X.; Si, C.; Du, H.; et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Hung, C.-L.; Chen, M.; Sohaimy, M.I.H.; Isa, M.I.N. Proton-Conducting Biopolymer Electrolytes Based on Carboxymethyl Cellulose Doped with Ammonium Formate. Polymers 2022, 14, 3019. [Google Scholar] [CrossRef]
- Liu, X.; Fan, X.; Liu, B.; Ding, J.; Deng, Y.; Han, X.; Zhong, C.; Hu, W. Mapping the Design of Electrolyte Materials for Electrically Rechargeable Zinc–Air Batteries. Adv. Mater. 2021, 33, 2006461. [Google Scholar] [CrossRef]
- Munaoka, T.; Yan, X.; Lopez, J.; To, J.W.F.; Park, J.; Tok, J.B.H.; Cui, Y.; Bao, Z. Ionically Conductive Self-Healing Binder for Low Cost Si Microparticles Anodes in Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703138. [Google Scholar] [CrossRef]
- Cai, M.; Park, S. Spectroelectrochemical Studies on Dissolution and Passivation of Zinc Electrodes in Alkaline Solutions. J. Electrochem. Soc. 1996, 143, 2125–2131. [Google Scholar] [CrossRef]
- Kumar, G.G.; Sampath, S. Electrochemical characterization of poly(vinylidenefluoride)-zinc triflate gel polymer electrolyte and its application in solid-state zinc batteries. Solid State Ion. 2003, 160, 289–300. [Google Scholar] [CrossRef]
- Girish Kumar, G.; Sampath, S. Electrochemical and spectroscopic investigations of a gel polymer electrolyte of poly(methylmethacrylate) and zinc triflate. Solid State Ion. 2005, 176, 773–780. [Google Scholar] [CrossRef]
- Tafur, J.P.; Romero, A.J.F. Interaction between Zn2+ cations and n-methyl-2-pyrrolidone in ionic liquid-based Gel Polymer Electrolytes for Zn batteries. Electrochim. Acta 2015, 176, 1447–1453. [Google Scholar] [CrossRef]
- Béjar, J.; Álvarez-Contreras, L.; Ledesma-García, J.; Arjona, N.; Arriaga, L.G. An advanced three-dimensionally ordered macroporous NiCo2O4 spinel as a bifunctional electrocatalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2020, 8, 8554–8565. [Google Scholar] [CrossRef]
- Díaz-Patiño, L.; Béjar, J.; Ortiz-Ortega, E.; Trejo, G.; Guerra-Balcázar, M.; Arjona, N.; Álvarez-Contreras, L. Zinc-Air Battery Operated with Modified-Zinc Electrodes/Gel Polymer Electrolytes. ChemElectroChem 2022, 9, e202200222. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Xu, N.; Peng, L.; Mao, J.; Gong, Q.; Qiao, J. Alkaline Exchange Polymer Membrane Electrolyte for High Performance of All-Solid-State Electrochemical Devices. ACS Appl. Mater. Interfaces 2018, 10, 29593–29598. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Song, X.; Jiang, G.; Zarrin, H.; Xu, P.; Li, K.; Yu, A.; Chen, Z. Laminated Cross-Linked Nanocellulose/Graphene Oxide Electrolyte for Flexible Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2016, 6, 1600476. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Liu, X.; Qu, S.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. Long-battery-life flexible zinc–air battery with near-neutral polymer electrolyte and nanoporous integrated air electrode. J. Mater. Chem. A 2019, 7, 25449–25457. [Google Scholar] [CrossRef]
- Poosapati, A.; Negrete, K.; Thorpe, M.; Hutchison, J.; Zupan, M.; Lan, Y.; Madan, D. Safe and flexible chitosan-based polymer gel as an electrolyte for use in zinc-alkaline based chemistries. J. Appl. Polym. Sci. 2021, 138, 50813. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Deng, H. Ion-conducting polymer gels of polyacrylamide embedded with K2CO3. J. Appl. Polym. Sci. 2004, 92, 2076–2081. [Google Scholar] [CrossRef]
- Ng, P.L.; Jamaludin, A.; Alias, Y.; Basirun, W.J.; Ahmad, Z.A.; Mohamad, A.A. Effect of KOH concentration in the gel polymer electrolyte for direct borohydride fuel cell. J. Appl. Polym. Sci. 2012, 123, 2662–2666. [Google Scholar] [CrossRef]










| Electrolyte | Hydrogel Code |
|---|---|
| CMC90/CS30 | CMC-CS |
| CMC90/CS30/CA30 | CA30 |
| CMC90/CS30/CA40 | CA40 |
| CMC90/CS30/CA50 | CA50 |
| CMC90/CS30 “sw” | CMC-CS sw |
| CMC90/CS30/CA30 “sw” | CA30 sw |
| CMC90/CS30/CA40 “sw” | CA40 sw |
| CMC90/CS30/CA50 “sw” | CA50 sw |
| Membranes | Xc (%) |
|---|---|
| CMC–CS | 17.9 |
| CA30 | 15.5 |
| CA40 | 14.4 |
| CA50 | 15.6 |
| CMC–CS sw | 10.7 |
| CA30 sw | 5.2 |
| CA40 sw | 4.6 |
| CA50 sw | 4.7 |
| Electrolyte | KOH Absorption (%) | Ea (eV) | σ (S∙cm−1) |
|---|---|---|---|
| CMC-CS sw | 288.35 ± 26.64 | 0.21 | 0.11 |
| CA30 sw | 180.40 ± 17.01 | 0.18 | 0.16 |
| CA40 sw | 160.83 ± 7.10 | 0.14 | 0.18 |
| CA50 sw | 151.69 ± 1.09 | 0.16 | 0.19 |
| Electrolyte | Ionic Conductivity (S cm−1) | Bulk Resistance (Ω) | Specific Capacitance (mA∙h g−1) | Power Density (mW cm−2) | Reference |
|---|---|---|---|---|---|
| Chitosan-PDDA-GA KOH | 0.02 | ∼1.00 | - | 48.9 | [98] |
| QA-functionalized nanocellulose-GO-KOH | 0.04 | - | - | 44.1 | [99] |
| PVA-PEG-SiO2 KOH | 0.06 | ∼1.30 | 720.6 | 62.6 | [100] |
| PVA-NH4Cl-ZnCl | 0.07 | 2.16 | - | ∼8 | [101] |
| CS-PVA KOH | 0.11 | 1.06 | 221.6 | - | [102] |
| CMC-CS-CA KOH | 0.19 | 1.85 | 1026 | 85 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bósquez-Cáceres, M.F.; Lima, L.D.; Morera Córdova, V.; Delgado, A.D.; Béjar, J.; Arjona, N.; Álvarez-Contreras, L.; Tafur, J.P. Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc–Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices. Batteries 2022, 8, 265. https://doi.org/10.3390/batteries8120265
Bósquez-Cáceres MF, Lima LD, Morera Córdova V, Delgado AD, Béjar J, Arjona N, Álvarez-Contreras L, Tafur JP. Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc–Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices. Batteries. 2022; 8(12):265. https://doi.org/10.3390/batteries8120265
Chicago/Turabian StyleBósquez-Cáceres, María Fernanda, Lola De Lima, Vivian Morera Córdova, Anabel D. Delgado, José Béjar, Noé Arjona, Lorena Álvarez-Contreras, and Juan P. Tafur. 2022. "Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc–Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices" Batteries 8, no. 12: 265. https://doi.org/10.3390/batteries8120265
APA StyleBósquez-Cáceres, M. F., Lima, L. D., Morera Córdova, V., Delgado, A. D., Béjar, J., Arjona, N., Álvarez-Contreras, L., & Tafur, J. P. (2022). Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc–Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices. Batteries, 8(12), 265. https://doi.org/10.3390/batteries8120265