Fabrication and Characterization of Plasma Sprayed TiO2 and Li4Ti5O12 Materials as All Active Material Lithium-Ion Battery Electrodes
Abstract
:1. Introduction
2. Materials and Methods
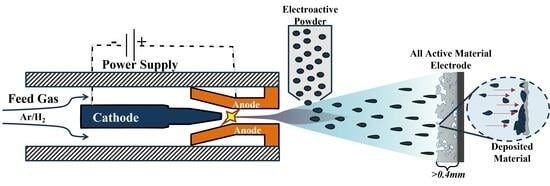
2.1. Plasma Spray Electrode Fabrication
2.2. Material Characterization
2.3. AAM Electrode Fabrication
2.4. Electrochemical Characterization
3. Results
3.1. TiO2 Electrodes
3.2. Li4Ti5O12 Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koenig, G.M.; Liu, N. Lithium-Ion Batteries and Their Impact on Our Lives. Electrochem. Soc. Interface 2022, 31, 55. [Google Scholar] [CrossRef]
- Sumboja, A.; Liu, J.; Zheng, W.G.; Zong, Y.; Zhang, H.; Liu, Z. Electrochemical Energy Storage Devices for Wearable Technology: A Rationale for Materials Selection and Cell Design. Chem. Soc. Rev. 2018, 47, 5919–5945. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-Dimensional Hierarchically Porous Nitrogen-Doped Carbon from Water Hyacinth as Selenium Host for High-Performance Lithium–Selenium Batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-Performance Monoclinic WO3 Nanospheres with the Novel NH4+ Diffusion Behaviors for Aqueous Ammonium-Ion Batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future Generations of Cathode Materials: An Automotive Industry Perspective. J. Mater. Chem. A Mater. 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Qi, Z.; Koenig, G.M. Review Article: Flow Battery Systems with Solid Electroactive Materials. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 040801. [Google Scholar] [CrossRef]
- Lai, W.; Erdonmez, C.K.; Marinis, T.F.; Bjune, C.K.; Dudney, N.J.; Xu, F.; Wartena, R.; Chiang, Y.M. Ultrahigh-Energy-Density Microbatteries Enabled by New Electrode Architecture and Micropackaging Design. Adv. Mater. 2010, 22, E139–E144. [Google Scholar] [CrossRef]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-Tortuosity and Graded Lithium Ion Battery Cathodes by Ice Templating. J. Mater. Chem. A Mater. 2019, 7, 21421–21431. [Google Scholar] [CrossRef]
- Li, L.; Erb, R.M.; Wang, J.; Wang, J.; Chiang, Y.M. Fabrication of Low-Tortuosity Ultrahigh-Area-Capacity Battery Electrodes through Magnetic Alignment of Emulsion-Based Slurries. Adv. Energy Mater. 2019, 9, 1802472. [Google Scholar] [CrossRef]
- Wu, J.; Ju, Z.; Zhang, X.; Quilty, C.; Takeuchi, K.J.; Bock, D.C.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Ultrahigh-Capacity and Scalable Architected Battery Electrodes via Tortuosity Modulation. ACS Nano 2021, 15, 19109–19118. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Djenizian, T.; Hanzu, I.; Knauth, P. Nanostructured Negative Electrodes Based on Titania for Li-Ion Microbatteries. J. Mater. Chem. 2011, 21, 9925–9937. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and Titania Nanostructured Materials for Environmental and Energy Applications: A Review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Cheng, Y.J.; Xia, Y.; Müller-Buschbaum, P. Anatase Titanium Dioxide as Rechargeable Ion Battery Electrode—A Chronological Review. Energy Storage Mater. 2022, 45, 201–264. [Google Scholar] [CrossRef]
- Koudriachova, M.V.; Harrison, N.M.; de Leeuw, S.W. Density-Functional Simulations of Lithium Intercalation in Rutile. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 65, 2354231. [Google Scholar] [CrossRef]
- Willenberg, L.K.; Dechent, P.; Fuchs, G.; Sauer, D.U.; Figgemeier, E. High-Precision Monitoring of Volume Change of Commercial Lithium-Ion Batteries by Using Strain Gauges. Sustainability 2020, 12, 557. [Google Scholar] [CrossRef]
- Parekh, M.H.; Sediako, A.D.; Naseri, A.; Thomson, M.J.; Pol, V.G. In Situ Mechanistic Elucidation of Superior Si-C-Graphite Li-Ion Battery Anode Formation with Thermal Safety Aspects. Adv. Energy Mater. 2020, 10, 1902799. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in Tio2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Wen, L.; Wang, C.; Zhao, H.; Mi, Y.; Liang, L.; Fu, Q.; Wu, M.; Lei, Y. Highly Ordered Three-Dimensional Ni-TiO2 Nanoarrays as Sodium Ion Battery Anodes. Chem. Mater. 2015, 27, 4274–4280. [Google Scholar] [CrossRef]
- Dambournet, D.; Belharouak, I.; Amine, K. Tailored Preparation Methods of TiO2 Anatase, Rutile, Brookite: Mechanism of Formation and Electrochemical Properties. Chem. Mater. 2010, 22, 1173–1179. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Djenizian, T.; Lavela, P.; Tirado, J.L.; Knauth, P. Alternative Li-Ion Battery Electrode Based on Self-Organized Titania Nanotubes. Chem. Mater. 2009, 21, 63–67. [Google Scholar] [CrossRef]
- Mukai, K.; Kato, Y.; Nakano, H. Understanding the Zero-Strain Lithium Insertion Scheme of Li[Li 1/3Ti5/3]O4: Structural Changes at Atomic Scale Clarified by Raman Spectroscopy. J. Phys. Chem. C 2014, 118, 2992–2999. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Rauhala, T.; Valkeapää, M.; Kallioinen, J.; Kallio, T. Effect of Li4Ti5O12 Particle Size on the Performance of Lithium Ion Battery Electrodes at High C-Rates and Low Temperatures. J. Phys. Chem. C 2015, 119, 2277–2283. [Google Scholar] [CrossRef]
- Miranda, D.; Almeida, A.M.; Lanceros-Méndez, S.; Costa, C.M. Effect of the Active Material Type and Battery Geometry on the Thermal Behavior of Lithium-Ion Batteries. Energy 2019, 185, 1250–1262. [Google Scholar] [CrossRef]
- Song, J.; Bazant, M.Z. Effects of Nanoparticle Geometry and Size Distribution on Diffusion Impedance of Battery Electrodes. J. Electrochem. Soc. 2013, 160, A15–A24. [Google Scholar] [CrossRef]
- Mayer, J.K.; Almar, L.; Asylbekov, E.; Haselrieder, W.; Kwade, A.; Weber, A.; Nirschl, H. Influence of the Carbon Black Dispersing Process on the Microstructure and Performance of Li-Ion Battery Cathodes. Energy Technol. 2020, 8, 1900161. [Google Scholar] [CrossRef]
- Jouhara, A.; Dupré, N.; Guyomard, D.; Lakraychi, A.E.; Dolhem, F.; Poizot, P. Playing with the P-Doping Mechanism to Lower the Carbon Loading in n-Type Insertion Organic Electrodes: First Feasibility Study with Binder-Free Composite Electrodes. J. Electrochem. Soc. 2020, 167, 070540. [Google Scholar] [CrossRef]
- Palomares, V.; Goñi, A.; De Muro, I.G.; De Meatza, I.; Bengoechea, M.; Cantero, I.; Rojo, T. Conductive Additive Content Balance in Li-Ion Battery Cathodes: Commercial Carbon Blacks vs. in Situ Carbon from LiFePO4/C Composites. J. Power Sources 2010, 195, 7661–7668. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Arias, A.C. Understanding the Effects of Electrode Formulation on the Mechanical Strength of Composite Electrodes for Flexible Batteries. ACS Appl. Mater. Interfaces 2017, 9, 6390–6400. [Google Scholar] [CrossRef]
- Ligneel, E.; Lestriez, B.; Guyomard, D. Relationships between Processing, Morphology and Discharge Capacity of the Composite Electrode. J. Power Sources 2007, 174, 716–719. [Google Scholar] [CrossRef]
- Foreman, E.; Zakri, W.; Hossein Sanatimoghaddam, M.; Modjtahedi, A.; Pathak, S.; Kashkooli, A.G.; Garafolo, N.G.; Farhad, S. A Review of Inactive Materials and Components of Flexible Lithium-Ion Batteries. Adv. Sustain. Syst. 2017, 1, 1700061. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Wang, F.; Lee, J.; Chen, L.; Zhang, G.; He, S.; Han, J.; Ahn, J.; Cheong, J.Y.; Jiang, S.; Kim, I.D. Inspired by Wood: Thick Electrodes for Supercapacitors. ACS Nano 2023, 17, 8866–8898. [Google Scholar] [CrossRef]
- Elango, R.; Demortière, A.; De Andrade, V.; Morcrette, M.; Seznec, V. Thick Binder-Free Electrodes for Li–Ion Battery Fabricated Using Templating Approach and Spark Plasma Sintering Reveals High Areal Capacity. Adv. Energy Mater. 2018, 8, 1703031. [Google Scholar] [CrossRef]
- Curry, N.; Leitner, M.; Körner, K. High-Porosity Thermal Barrier Coatings from High-Power Plasma Spray Equipment—Processing, Performance and Economics. Coatings 2020, 10, 957. [Google Scholar] [CrossRef]
- Chang, S.M.; Rodríguez Tolava, E.F.; Yang, Y.J.; Li, H.C.; Lee, R.C.; Wu, N.L.; Hsu, C.C. One-Step Fast Synthesis of Li 4 Ti 5 O 12 Particles Using an Atmospheric Pressure Plasma Jet. J. Am. Ceram. Soc. 2014, 97, 708–712. [Google Scholar] [CrossRef]
- Hsueh, T.-H.; Tsai, C.-H.; Liu, S.-E.; Wang, M.-C.; Chang, S.-M.; Shiue, A.; Chin, K.-Y. LiCoO 2 Battery Electrode Fabricated by High Deposition-Rate Atmospheric Plasma Spraying for Lithium Battery. J. Electrochem. Soc. 2022, 169, 100506. [Google Scholar] [CrossRef]
- Wu, X.; Liang, X.; Zhang, X.; Lan, L.; Li, S.; Gai, Q. Structural Evolution of Plasma Sprayed Amorphous Li4Ti5O12 Electrode and Ceramic/Polymer Composite Electrolyte during Electrochemical Cycle of Quasi-Solid-State Lithium Battery. J. Adv. Ceram. 2021, 10, 347–354. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Y.; Zhang, X.; Han, D.; Lan, L.; Zhang, Y. Performance Study of a Li4Ti5O12 Electrode for Lithium Batteries Prepared by Atmospheric Plasma Spraying. Ceram. Int. 2019, 45, 23750–23755. [Google Scholar] [CrossRef]
- Robinson, J.P.; Ruppert, J.J.; Dong, H.; Koenig, G.M. Sintered Electrode Full Cells for High Energy Density Lithium-Ion Batteries. J. Appl. Electrochem. 2018, 48, 1297–1304. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 Spinel Anode: Fundamentals and Advances in Rechargeable Batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Gardon, M.; Guilemany, J.M. Milestones in Functional Titanium Dioxide Thermal Spray Coatings: A Review. J. Therm. Spray Technol. 2014, 23, 577–595. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M.; Ma, X. Dense TiO2 Coating Using the Solution Precursor Plasma Spray Process. Proc. J. Am. Ceram. Soc. 2008, 91, 865–872. [Google Scholar] [CrossRef]
- Yang, G.J.; Li, C.J.; Han, F.; Li, W.Y.; Ohmori, A. Low Temperature Deposition and Characterization of TiO 2 Photocatalytic Film through Cold Spray. Appl. Surf. Sci. 2008, 254, 3979–3982. [Google Scholar] [CrossRef]
- Herrmann-Geppert, I.; Bogdanoff, P.; Emmler, T.; Dittrich, T.; Radnik, J.; Klassen, T.; Gutzmann, H.; Schieda, M. Cold Gas Spraying—A Promising Technique for Photoelectrodes: The Example TiO2. Catal. Today 2016, 260, 140–147. [Google Scholar] [CrossRef]
- Winnicki, M.; Baszczuk, A.; Jasiorski, M.; Borak, B.; Małachowska, A. Preliminary Studies of TiO2 Nanopowder Deposition onto Metallic Substrate by Low Pressure Cold Spraying. Surf. Coat. Technol. 2019, 371, 194–202. [Google Scholar] [CrossRef]
- Stanford, M.K.; DellaCorte, C.; Eylon, D. Particle Size Effects on Flow Properties of PS304 Plasma Spray Feedstock Powder Blend. In 27th Annual Cocoa Beach Conference on Advanced Ceramics and Composites: A: Ceramic Engineering and Science Proceedings; Ceramic Engineering and Science Proceeding (CESP); American Ceramic Society: Westerville, OH, USA, 2003; Volume 24, pp. 577–586. [Google Scholar]
- Vardelle, A.; Moreau, C.; Themelis, N.J.; Chazelas, C. A Perspective on Plasma Spray Technology. Plasma Chem. Plasma Process. 2015, 35, 491–509. [Google Scholar] [CrossRef]
- Shin, J.Y.; Samuelis, D.; Maier, J. Sustained Lithium-Storage Performance of Hierarchical, Nanoporous Anatase TiO2 at High Rates: Emphasis on Interfacial Storage Phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Vu, A.; Qian, Y.; Stein, A. Porous Electrode Materials for Lithium-Ion Batteries-How to Prepare Them and What Makes Them Special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Zlámalová, M.; Pitňa Lásková, B.; Vinarčíková, M.; Zukalová, M.; Kavan, L. Inherent Electrochemical Activity of TiO2 (Anatase, Rutile) Enhances the Charge Capacity of Cathodes of Lithium-Sulfur Batteries. J. Solid State Electrochem. 2022, 26, 639–647. [Google Scholar] [CrossRef]
- Smith, S.J.; Stevens, R.; Liu, S.; Li, G.; Navrotsky, A.; Boerio-Goates, J.; Woodfield, B.F. Heat Capacities and Thermodynamic Functions of TiO2 Anatase and Rutile: Analysis of Phase Stability. Am. Mineral. 2009, 94, 236–243. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO 2 Powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- Kubiak, P.; Fröschl, T.; Hüsing, N.; Hörmann, U.; Kaiser, U.; Schiller, R.; Weiss, C.K.; Landfester, K.; Wohlfahrt-Mehrens, M. TiO2 Anatase Nanoparticle Networks: Synthesis, Structure, and Electrochemical Performance. Small 2011, 7, 1690–1696. [Google Scholar] [CrossRef]
- Nie, Z.; Parai, R.; Cai, C.; Michaelis, C.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L.; Ghosh, D.; Koenig, G.M. Pore Microstructure Impacts on Lithium Ion Transport and Rate Capability of Thick Sintered Electrodes. J. Electrochem. Soc. 2021, 168, 060550. [Google Scholar] [CrossRef]
- Cai, C.; Nie, Z.; Koenig, G.M. Multicomponent Two-Layered Cathode for Thick Sintered Lithium-Ion Batteries. Mater. Adv. 2022, 3, 4200–4212. [Google Scholar] [CrossRef]
- Fischer, M.G.; Hua, X.; Wilts, B.D.; Gunkel, I.; Bennett, T.M.; Steiner, U. Mesoporous Titania Microspheres with Highly Tunable Pores as an Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 22388–22397. [Google Scholar] [CrossRef]
- Cai, C.; Hensley, D.; Koenig, G.M. Simulated Discharge Overpotential Distributions for Sintered Electrode Batteries in Rechargeable Coin Cell Form Factors. J. Energy Storage 2022, 54, 105218. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Van Eck, E.R.H.; Kentgens, A.P.M.; Kearley, G.J.; Mulder, F.M. The Influence of Size on Phase Morphology and Li-Ion Mobility in Nanosized Lithiated Anatase TiO2. Chem. Eur. J. 2007, 13, 2023–2028. [Google Scholar] [CrossRef]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.A.; Said, N.D.M.; Abdullah, S.A.; Rahim, M.S. Effect of Annealing Temperature of Titanium Dioxide Thin Films on Structural and Electrical Properties. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2017; Volume 1788. [Google Scholar]
- Deng, W.; Liu, W.; Zhu, H.; Chen, L.; Liao, H.; Chen, H. Click-Chemistry and Ionic Cross-Linking Induced Double Cross-Linking Ionogel Electrolyte for Flexible Lithium-Ion Batteries. J. Energy Storage 2023, 72, 108509. [Google Scholar] [CrossRef]
- Moon, E.J.; Hong, J.K.; Mohanty, S.K.; Yang, M.; Ihm, K.; Lee, H.; Yoo, H.D. Coating Lithium Titanate Anodes with a Mixed Ionic-Electronic Conductor for High-Rate Lithium-Ion Batteries. J. Power Sources 2023, 559, 232657. [Google Scholar] [CrossRef]
- Cai, C.; Yost, D.; Koenig, G.M. Increased Cycling Rates for Thick All Active Material Electrodes via Electrolyte Modifications. J. Energy Storage 2023, 64, 107238. [Google Scholar] [CrossRef]
- Cai, C.; Koenig, G.M. Enhancing Low Electronic Conductivity Materials in All Active Material Electrodes through Multicomponent Architecture. Energy Adv. 2023, 2, 308–320. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; Torre-Gamarra, C.; de la Levenfeld, B.; Sanchez, J.Y.; Varez, A.; Kim, G.T.; Varzi, A.; Passerini, S. Ultra-Thick Battery Electrodes for High Gravimetric and Volumetric Energy Density Li-Ion Batteries. J. Power Sources 2019, 437, 226923. [Google Scholar] [CrossRef]
- Deng, W.; Shi, W.; Liu, Q.; Jiang, J.; Li, X.; Feng, X. Constructing Gradient Porous Structure in Thick Li4Ti5O12 Electrode for High-Energy and Stable Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 17062–17068. [Google Scholar] [CrossRef]
- Parai, R.; Nie, Z.; Ghosh, D.; Koenig, G.M. Microstructure and Mechanical Properties of Electrochemically Cycled Ice-Templated Li4Ti5O12 Sintered Anodes. Int. J. Energy Res. 2022, 46, 11501–11509. [Google Scholar] [CrossRef]
- Lin, Y.S.; Duh, J.G. Facile Synthesis of Mesoporous Lithium Titanate Spheres for High Rate Lithium-Ion Batteries. J. Power Sources 2011, 196, 10698–10703. [Google Scholar] [CrossRef]
- Lin, J.Y.; Hsu, C.C.; Ho, H.P.; Wu, S.H. Sol-Gel Synthesis of Aluminum Doped Lithium Titanate Anode Material for Lithium Ion Batteries. Electrochim. Acta 2013, 87, 126–132. [Google Scholar] [CrossRef]
- Narayana, L.; Hussain, O.M.; Mauger, A.; Julien, C.M. Transport Properties of Nanostructured Li2TiO3 Anode Material Synthesized by Hydrothermal Method. Science 2019, 1, 39. [Google Scholar] [CrossRef]
- Marthi, R.; Asgar, H.; Gadikota, G.; Smith, Y.R. On the Structure and Lithium Adsorption Mechanism of Layered H2TiO3. ACS Appl. Mater. Interfaces 2021, 13, 8361–8369. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, H.; Xue, X.; Cao, S.; Cao, H.; Shi, L. Synthesizing Nano-Sized (∼20 Nm) Li4Ti5O12 at Low Temperature for a High-Rate Performance Lithium Ion Battery Anode. RSC Adv. 2014, 4, 63105–63109. [Google Scholar] [CrossRef]
- Ge, H.; Li, N.; Li, D.; Dai, C.; Wang, D. Study on the Theoretical Capacity of Spinel Lithium Titanate Induced by Low-Potential Intercalation. J. Phys. Chem. C 2009, 113, 6324–6326. [Google Scholar] [CrossRef]
- Lu, X.; Gu, L.; Hu, Y.S.; Chiu, H.C.; Li, H.; Demopoulos, G.P.; Chen, L. New Insight into the Atomic-Scale Bulk and Surface Structure Evolution of Li4Ti5O12 Anode. J. Am. Chem. Soc. 2015, 137, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lochala, J.; Taverne, T.; Xiao, J. The Interplay between Solid Electrolyte Interface (SEI) and Dendritic Lithium Growth. Nano Energy 2017, 40, 34–41. [Google Scholar] [CrossRef]
- Wang, H.; Matsui, M.; Kuwata, H.; Sonoki, H.; Matsuda, Y.; Shang, X.; Takeda, Y.; Yamamoto, O.; Imanishi, N. A Reversible Dendrite-Free High-Areal-Capacity Lithium Metal Electrode. Nat. Commun. 2017, 8, 15106. [Google Scholar] [CrossRef]
- Nie, Z.; Ong, S.; Hussey, D.S.; Lamanna, J.M.; Jacobson, D.L.; Koenig, G.M. Probing Transport Limitations in Thick Sintered Battery Electrodes with Neutron Imaging. Mol. Syst. Des. Eng. 2020, 5, 245–256. [Google Scholar] [CrossRef]
- Nie, Z.; Parai, R.; Cai, C.; Ghosh, D.; Koenig, G.M. Improving High Rate Cycling Limitations of Thick Sintered Battery Electrodes by Mitigating Molecular Transport Limitations through Modifying Electrode Microstructure and Electrolyte Conductivity. Mol. Syst. Des. Eng. 2021, 6, 708–712. [Google Scholar] [CrossRef]
- Young, D.; Ransil, A.; Amin, R.; Li, Z.; Chiang, Y.M. Electronic Conductivity in the Li4/3Ti5/3O 4-Li7/3Ti5/3O4 System and Variation with State-of-Charge as a Li Battery Anode. Adv. Energy Mater. 2013, 3, 1125–1129. [Google Scholar] [CrossRef]
- Nikolian, A.; Fleurbaey, K.; Timmermans, J.-M.; De Hoog, J.; Fleurbay, K.; Noshin, O.; Van De Bossche, P.; Van Mierlo, J. Classification of electric modeling and characterization methods of lithium-ion batteries for vehicle applications. In Proceedings of the European Electric Vehicle Congress 2014, Brussels, Belgium, 2–5 December 2014. [Google Scholar]






| Parameter | LTO | TiO2 |
|---|---|---|
| Standoff Distance [mm] | 120 | 150 |
| Surface Speed [mm s−1] | 1250 | 1250 |
| Step Size [mm] | 3 | 3 |
| Parameter | TiO2 | LTO (High Intensity) | LTO (Low Intensity) |
|---|---|---|---|
| Jambox Voltage [V] | 70 | 78 | 55 |
| Gun Current [A] | 330 | 230 | 200 |
| Gun Powder [kW] | 23 | 18 | 11 |
| Argon Flow (Primary) [×10−3 m3 s−1] | 0.783 | 1.50 | 1.33 |
| Hydrogen Flow (secondary) [×10−3 m3 s−1] | 0.200 | 0.200 | 0.030 |
| Powder Feed Rate [g min−1] | 20 | 15 | 15 |
| Polyester Feed Rate [g min−1] | 5 | 4 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yost, D.; Laurer, J.; Childrey, K.; Cai, C.; Koenig, G.M., Jr. Fabrication and Characterization of Plasma Sprayed TiO2 and Li4Ti5O12 Materials as All Active Material Lithium-Ion Battery Electrodes. Batteries 2023, 9, 598. https://doi.org/10.3390/batteries9120598
Yost D, Laurer J, Childrey K, Cai C, Koenig GM Jr. Fabrication and Characterization of Plasma Sprayed TiO2 and Li4Ti5O12 Materials as All Active Material Lithium-Ion Battery Electrodes. Batteries. 2023; 9(12):598. https://doi.org/10.3390/batteries9120598
Chicago/Turabian StyleYost, Dean, Jonathan Laurer, Kevin Childrey, Chen Cai, and Gary M. Koenig, Jr. 2023. "Fabrication and Characterization of Plasma Sprayed TiO2 and Li4Ti5O12 Materials as All Active Material Lithium-Ion Battery Electrodes" Batteries 9, no. 12: 598. https://doi.org/10.3390/batteries9120598
APA StyleYost, D., Laurer, J., Childrey, K., Cai, C., & Koenig, G. M., Jr. (2023). Fabrication and Characterization of Plasma Sprayed TiO2 and Li4Ti5O12 Materials as All Active Material Lithium-Ion Battery Electrodes. Batteries, 9(12), 598. https://doi.org/10.3390/batteries9120598