1. Introduction
Silicon-based anodes are the most promising Li-ion batteries due to their high theoretical capacities (over 3000 mAh/g compared to 372 mAh/g for graphite) and abundance in nature; however, they undergo colossal volume expansions of approximately 400% upon maximum lithiation [
1,
2,
3,
4,
5,
6,
7]. This leads to severe damage and fracture, which reduces the active Si that can react with Li; therefore, the initial experimental capacity (>3000 mAh/g) [
8] is significantly reduced during electrochemical cycling. Experimental [
9,
10] and theoretical [
10,
11] studies have shown that reducing the Si particle size below 100 nm can prevent damage of the individual particles in porous-type electrodes (Si + binder + additives). This fracture alleviation can interpret the very promising capacity retentions that have been achieved for nanoscale Si-based anodes, which range between 750 mAh/g for 1000 cycles [
12], 1271 mAh/g for 1000 cycles [
13], and 2312 mAh/g for 100 cycles [
14]. In situ lithiation of individual Si particles has shown that fracture can be avoided for 380 nm Si particles (i.e., greater than 100 nm) [
15]; however, the conditions in such cases are very different than those present in porous electrodes.
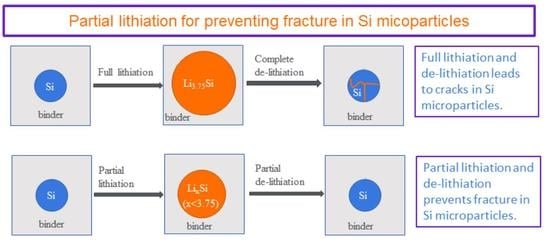
Mass producing nano-Si-based porous electrodes for the plethora applications of Li-ion batteries is prohibitive not only due to the cost of nanoparticles, but also the difficulty in preventing agglomeration during their fabrication and storage. Battery companies recently have begun commercializing Si-based anodes that contain very low Si contents with a particle size over 500 nm in order for the interparticle spacing to be large enough and have the binder act as a buffering agent to prevent fracture. Tuning the binder and interparticle spacing is a key factor in preventing fracture as earlier theoretical studies have shown [
16]; however, another aspect that controls damage is the lithiation state. It was shown that full discharge of SiO particles at a C-rate of 1/24 led to the cracking of the particles with diameters greater than 2 µm near the surface, and pulverization of the particles with diameters greater than 10 µm [
4]. Although partial lithiation of the silicon particles results in lower capacities (as the complete Si particle is not utilized), it corresponds to smaller volume changes (and hence damage) of the Si, which can result in electrochemical longevity, as numerous studies have shown [
17,
18,
19,
20,
21]. Recently, it was illustrated that partial lithiation of Si microparticles in porous electrodes allowed for a significantly improved performance over 250 full cycles [
22]. It was suggested that the improved performance was related to the reduction in mechanical stresses with partial lithiation; however, a more recent study indicated that partial lithiation also allowed the retention of Si crystallinity, which can also improve cyclability [
23].
In order to fully understand how to utilize partial lithiation, it is necessary to probe its relationship to Si damage and fracture during cycling. From an electrochemical point of view, it was shown experimentally that for 4.5 µm Si-particle porous electrodes, the partial lithiation should not exceed 30% in order to attain a stable capacity for 250 full cycles [
22]. It was assumed that damage increased at higher lithiation states resulting in the observed capacity decay; however, only a single scanning electron microscopy image was provided showing a very small area of Si to support this hypothesis. Herein, we therefore perform a numerical analysis in order to document the development of damage for different partial lithiation states for microscale Si particles embedded in a polymer. In order to study the effect of particle size and particle distribution (within the porous electrode) on fracture, four cases are considered: that of small and larger Si particles distributed within a polymer binder (the large Si particles are either 1 or 2 µm), and the case of only same size larger Si particles embedded in the binder (again with diameters of either 1 or 2 µm). This is the first study that provides a correlation between partial lithiation and damage that can aid battery developers in employing micron Si particles, without having to keep their volume fractions low. In addition to Si particles, this numerical model can be applied to other types of active particles (such as germanium or tin) that can be used in Li-ion and Na-ion batteries.
2. Modeling
In order to reduce computation time, a 2D (rather than 3D) representation will be employed. In this study, several square polymer binders embedded with multiple Si particles are considered. There are two different configurations. In the first case, there are five bigger Si particles and four smaller Si particles embedded in the polymer binder, in which the radii of the smaller particles are only one half of the larger ones. In the second case, the smaller particles are removed and only the larger ones are considered. To start with, a square polymer binder of 6400 nm × 6400 nm with both small and large particles is considered, with diameters of 500 nm and 1 µm, respectively. The spacing between two adjacent 1 µm particles (in either the horizontal or vertical directions) is 1600 nm. To evaluate the damage evolution of this 2D configuration during lithiation, a phase field damage model coupled with the diffusion equation with a nonlinear concentration-dependent diffusivity is adopted [
10,
24,
25]. This model has been experimentally verified as it was successfully applied to capture the dry lake-bed fracture pattern in Si thin-film electrodes [
24], the critical particle size of Si below which no fracture would occur during full lithiation [
10], and the composition of cathodes that would alleviate damage during sodiation [
25].
Lithium transport can be expressed by Fick’s law as [
10,
24,
25]:
where the concentration-dependent diffusivity parameter
D is given by
and
c* denotes the normalized/relative Li-ion concentration, equal to the actual molar concentration of Li-ions per unit volume divided by the maximum Li-ion concentration.
c* is dimensionless and can range from 0 to 1, corresponding to the fully de-lithiated state (pure Si) and completely lithiated state (Li
3.75Si at room temperature), respectively.
D0 is the initial diffusivity, when no Li-ions are present in the Si, i.e.,
c* = 0. As for the mechanical part, small deformation theory is adopted. The total strain
εt can be decomposed as the sum of the stress-free strain
εc which is induced by Li-ion diffusion and the elastic strain
εe:
where
I is the second-order unit tensor,
u is the displacement field,
α is the dilatation coefficient (analogy to thermal expansion coefficient) and a negative
α is needed for de-lithiation. The equilibrium equation (balance of linear momentum) is given by:
where
σ is the Cauchy stress tensor. Furthermore, the governing equation for the damage filed can be described by [
26]:
where the phase field variable
d can only grow from 0 to 1 (corresponding to the intact state and fully broken state, respectively),
Gc is material-dependent Griffith’s critical energy release rate (the energy required to create a fracture surface per unit area), and
l is an internal length scale characteristic of the material.
is a history variable that is the maximum elastic strain energy density over time. The initial conditions are concentration
c* = 0 and damage
d = 0 for lithiation. Similar to the dilatation coefficient, a negative ion flux is adopted for de-lithiation. In addition, the initial conditions for the concentration and damage field come from the results after full lithiation. The boundary conditions for this Li/electrode system are a constant flux of Li-ions (1.4 nm/ms [
24]) at the exterior surface of the electrode and the symmetry displacement boundary conditions for the outer surfaces of the polymer [
27]. The Young’s modulus of the Si particles is 130 GPa, while the polymer is taken to be composed of the common binder.
α is set as 0.3 [
24] for the Si electrode, while for the binder it is assumed to be zero, since it is taken to be inert with respect to Li and therefore cannot host Li-ions. The energy release rate for the Si electrodes was determined to be 10–12 J/m
2 [
28]. It should be noted that if the experimental value is adopted for
Gc, a much lower internal length is needed to obtain the desired fracture patterns. Thus, a more refined mesh is needed which is relatively computationally expensive. In the present study,
Gc for the Si particles is 50 J/m
2. The internal length is a material constant that depends on the microstructure and cannot be larger than the particle; hence, for the Si it is taken to be 50 nm. For an ideal Si-based electrode, the polymer surrounding the Si would not fracture, and in order for the model to capture this, the energy release rate for the polymer is taken to be 100 times higher than that of Si.
The governing equations are solved in the open-source software FEniCS by employing an unstructured mesh that has a size approximately 1/100 of the side length of the polymer. The Euler backward method is employed for the discretization of the diffusion equations and the time step size is chosen as 400 sec. It takes 110 steps (approximately 12 h) to complete a full lithiation or another 110 steps for complete de-lithiation. The full lithiation refers to the state of the battery in which the anode material of the battery exhibits its highest loading of lithium.
3. Results and Discussion
To investigate the relationship between partial lithiation and Si fracture, the damage evolution for different lithiation states was obtained. The concentration profiles provide the distance within the Si particle over which the Li-ions diffuse and therefore the percentage of Li within the Si was estimated for various partial lithiation states. To eliminate unrealistic damage, after lithiation and before de-lithiation, the value of damage is initialized to 0 except when it was higher than 0.9. This allows the electrode to store elastic energy again, avoiding the effect of damage values close to 0.5 that are somehow unrealistic. Damage profiles after full and partial lithiation and de-lithiation are shown in
Figure 1. It should be noted that for the case of partial lithiation, once the specified partial lithiation was reached, de-lithiation took place.
To investigate the relationship between partial lithiation and Si fracture, the damage evolution for different lithiation states was obtained. The concentration profiles provide the distance within the Si particle over which the Li-ions diffuse and therefore the percentage of Li within the Si was estimated for various partial lithiation states. To eliminate unrealistic damage, after lithiation and before de-lithiation, the value of damage was initialized to 0 except for when it was higher than 0.9. This allows the electrode to store elastic energy again, avoiding the effect of damage values close to 0.5 that are somehow unrealistic. Damage profiles after full and partial lithiation and de-lithiation are shown in
Figure 1. It should be noted that for the case of partial lithiation, once the specified partial lithiation was reached, de-lithiation took place.
Based on the simulation results, it is seen that cracks begin to initiate in the 1 µm particles after complete lithiation (110 time steps, i.e., 12.2 h) and continue to propagate during the de-lithiation process cutting the particles in half. This has been shown to be in agreement with experiments, as 1 µm Si particles in porous electrodes fractured in this manner upon complete lithiation [
10]. If the lithiation degree of the particle is 67% (104 time steps, i.e., 11.56 h), the damage after one cycle is less than 0.9, and can therefore be neglected. However, once the partial lithiation degree is greater than 72% (105 time steps, i.e., 11.67 h), it will lead to fracture in the 1
µm Si particles. In particular, it is seen that even if the damage is negligible, after 72% lithiation of the Si, once de-lithiation takes place at this partial lithiation state, fracture begins to occur in some particles.
To study the influence of particle size on the fracture pattern, the same configuration as shown in
Figure 1 is used but the sizes for both the polymer and particles are doubled, i.e., the size of the polymer binder is 12,800 nm × 12,800 nm and the radii of the smaller and larger particles are 1 µm and 2 µm, respectively. The ion flux is 1.2 nm/ms and the time step size is chosen as 800 s. The other parameters are the same as before. It takes 250 steps (approximately 55.6 h) to complete a full lithiation and another 250 steps for complete de-lithiation. Damage profiles after full and partial lithiation and de-lithiation are shown in
Figure 2. The damage profile (
Figure 2b) after full lithiation is also similar to the previous case (
Figure 1b) with smaller particle sizes since, again, there are four Si particles cut into halves. When the particle size is increased, however, more decohesion (i.e., detachment of the active material from the binder) takes place, as seen in
Figure 2. The damage profiles after partial lithiation and de-lithiation are also similar between the cases in
Figure 1 and
Figure 2; however, a different critical partial lithiation state to prevent fracture is noted.
Figure 2c–f show the damage profiles after 60% (212 time steps, i.e., 47.1 h) and 64% (213 time steps, i.e., 47.3 h) lithiation and de-lithiation, respectively. In particular, it is found that the critical lithiation degree for the configuration in
Figure 2 is 64%.
As mentioned, the lithiation states were estimated from the concentration profiles. This is illustrated in
Figure 3, which corresponds to the critical partial lithiation case where fracture began to take place upon de-lithiation. It is seen that when the radius of the large Si particles was 500 nm, fracture was fully prevented in the square domain of 6400 nm × 6400 nm when the Li-ion penetration did not exceed 360 nm, which corresponds to 72% lithiation of the particle (as shown in
Figure 3a). This means that limiting the lithiation state below this critical value can prevent fracture and crack growth in 1 µm Si particles, enhancing the electrochemical performance. It should be noted that the small Si particles of 500 nm did not experience damage for this lithiation state, illustrating that the lithiation state affecting damage is size dependent and must be considered separately for each particle size.
To have a better understanding of the configurations on the fracture pattern, more simulations were performed. Compared to the previous simulations (
Figure 1 and
Figure 2), the smaller particles are removed and only the larger ones are considered. All the other parameters are kept the same as in the previous cases. For the Si particles with 500 nm radii and square domain of 6400 nm × 6400 nm, the damage profiles are shown in
Figure 4. It takes 12 h to complete the full lithiation. The damage profiles after full lithiation and de-lithiation are shown in
Figure 4a,b, while
Figure 4c–f show those after 62% lithiation for 10 h and 66% lithiation for 10.2 h lithiation and de-lithiation, respectively.
In particular, it is found that the critical lithiation degree to prevent fracture for this configuration is 66%. Similarly, for the Si particles with a radius of 1 micron and square domain of 12,800 nm × 12,800 nm, the damage profiles are depicted in
Figure 5. It takes 54 h to complete the full lithiation. The damage profiles after full lithiation and de-lithiation are shown in
Figure 5a,b, while
Figure 5c–f show the damage profiles after 58% lithiation for 45.4 h and 60% lithiation for 45.6 h, respectively. In particular, it is found that the critical lithiation degree preventing fracture of the Si for this case is 60%. The concentration profiles for the critical lithiation states for
Figure 4 and
Figure 5 are plotted in
Figure 6, providing a maximum Li-ion concentration and the penetration depths of 320 mm and 580 mm to prevent fracture in geometries containing only large 1 micron and 2 micron Si particles, respectively.
By taking a closer look at these damage profiles, it can be concluded that the fracture pattern depends on the particle size, lithiation state, and the configuration of the Si binder system. An interesting finding is that when there are less particles present, cracks begin to initiate and propagate within the center of the particle. This is due to the fact that Li-ion diffusion is more difficult when the central particle is surrounded by smaller particles. As a result, the concentration gradient is less severe, leading to relatively less damages in the configurations containing a distribution of small and large Si particles. The present results are not complemented by respective experiments, as documenting morphology changes during partial lithiation is time consuming and tedious. However, the proposed design configurations are currently being considered experimentally and the results will be reported in the future.