Reclaiming the Value of Cotton Waste Textiles: A New Improved Method to Recycle Cotton Waste Textiles via Acid Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Cotton Waste Textiles
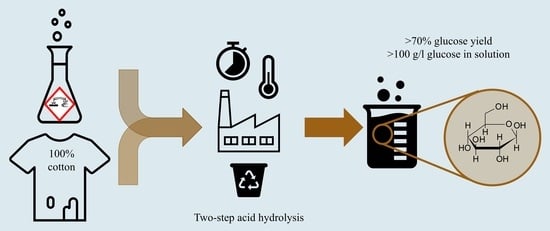
2.2. Two-Step Acid Hydrolysis
2.2.1. Pre-Hydrolysis
2.2.2. Post-Hydrolysis
2.3. Recyclability of the Solid Residue
2.4. Compositional Analysis of Cotton Waste Textiles and the Products following Two-Step Acid Hydrolysis
2.5. Collection, Two-Step Hydrolysis, and Compositional Analysis of Viscose Waste Textiles and Its Derivatives
2.6. Calculations
3. Results and Discussion
3.1. Composition of Cotton Waste Textiles
3.2. Optimization of the Two-Step Acid Hydrolysis
3.2.1. Pre-Hydrolysis
3.2.2. Optimization of the Post-Hydrolysis Step
3.3. Recyclability of Solid Residue
3.4. Product Requirement and Post-Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.-L.; Burns, L.D. Environmental Analysis of Textile Products. Cloth. Text. Res. J. 2006, 24, 248–261. [Google Scholar] [CrossRef]
- Pensupa, N.; Leu, S.-Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Lin, C.S.K. Recent Trends in Sustainable Textile Waste Recycling Methods: Current Situation and Future Prospects. Top. Curr. Chem. 2017, 375, 76. [Google Scholar] [CrossRef]
- Lanfranchi, M.; Cline, E.L. Cotton: A Case Study in Misinformation; Transformers Foundation: New York, NY, USA, 2021. [Google Scholar]
- Ruiz, L. Global Textile Fibre Demand: Trends and Forecast. 2019. Available online: https://icac.org/Content/EventDocuments/PdfFiles4407c817_a379_45b7_b3ac_c33809c9ae4d/4OS-Global%20Textile%20Fibres%20Demand-%20Trends%20and%20Forecast.pdf (accessed on 26 January 2021).
- Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion s Future; Ellen MacArthur Foundation: Cowes, UK, 2017. [Google Scholar]
- Textile Exchange. Preferred Fiber & Materials Market Report 2021; Pepper, L.R., Ed.; Textile Exchange: Lamesa, TX, USA, 2021. [Google Scholar]
- Renewcell. Recycling Clothes Finally Works. Available online: https://www.renewcell.com/en/2021 (accessed on 4 March 2021).
- David, S.K.; Pailthorpe, M.T. Classification of Textile Fibres: Production, Structure, and Properties. In Forensic Examination of Fibres; Robertson, J., Grieve, M., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–31. [Google Scholar]
- Wedin, H.; Lopes, M.H.; Sixta, H.; Hummel, M. Evaluation of post-consumer cellulosic textile waste for chemical recycling based on cellulose degree of polymerization and molar mass distribution. Text. Res. J. 2019, 89, 5067–5075. [Google Scholar] [CrossRef]
- Braconnot, H. Verwandlungen des Holzstoffs mittelst Schwefelsäure in Gummi, Zucker und eine eigne Säure, und mittelst Kali in Ulmin. Ann. Phys. 1819, 63, 347–371. [Google Scholar] [CrossRef]
- Freudenberg, K. The kinetics of long chain disintegration applied to cellulose and starch. Trans. Faraday Soc. 1936, 32, 74–75. [Google Scholar] [CrossRef]
- Battista, O.A. Hydrolysis and Crystallization of Cellulose. Ind. Eng. Chem. 1950, 42, 502–507. [Google Scholar] [CrossRef]
- Saeman, J.F. Kinetics of Wood Saccharification—Hydrolysis of Cellulose and Decomposition of Sugars in Dilute Acid at High Temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Sharples, A. The hydrolysis of cellulose and its relation to structure. Trans. Faraday Soc. 1957, 53, 1003–1013. [Google Scholar] [CrossRef]
- Farone, W.A.; Cuzens, J.E. Method of Producing Sugars Using Strong Acid Hydrolysis of Cellulosic and Hemicellulosic Materials. U.S. Patent 5,562,777, 8 October 1996. [Google Scholar]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Kong-Win Chang, J.; Duret, X.; Berberi, V.; Zahedi-Niaki, H.; Lavoie, J.-M. Two-Step thermochemical cellulose hydrolysis with partial neutralization for glucose production. Front. Chem. 2018, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Saeman, J.F. Key Factors in the Hydrolysis of Cellulose. In Biomass as a Nonfossil Fuel Source; ACS Publications: Washington, DC, USA, 1981; pp. 185–197. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- ISO 21436:2021, IDT; Pulps—Determination of Lignin Content—Acid Hydrolysis Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Jeihanipour, A.; Taherzadeh, M. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 2009, 100, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Sebastiá, M.; Ruuth, E.; Stigsson, L.; Galbe, M.; Wallberg, O. Novel sustainable alternatives for the fashion industry: A method of chemically recycling waste textiles via acid hydrolysis. Waste Manag. 2020, 121, 248–254. [Google Scholar] [CrossRef]
- Kihlman, M.; Aldaeus, F.; Chedid, F.; Germgård, U. Effect of various pulp properties on the solubility of cellulose in sodium hydroxide solutions. Holzforschung 2012, 66, 601–606. [Google Scholar] [CrossRef]
- Ouchi, A.; Toida, T.; Kumaresan, S.; Ando, W.; Kato, J. A new methodology to recycle polyester from fabric blends with cellulose. Cellulose 2010, 17, 215–222. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Novy, V.; Stigsson, L.; Galbe, M.; Wallberg, O. Towards circular fashion—Transforming pulp mills into hubs for textile recycling. RSC Adv. 2021, 11, 12321–12329. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- ISO 1833-1:2006/COR 1:2009; Textiles—Quantitative Chemical Analysis—Part 1: General Principles of Testing—Technical Corrigendum 1. International Organization for Standardization (ISO): Geneva, Switzerland, 2010.
- Sluiter, A.; Hyman, D.; Payne, C.; Wolfe, J. Determination of Insoluble Solids in Pretreated Biomass Material: Laboratory Analytical Procedure (LAP); NREL/TP-510-42627; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Saeman, J.F.; Moore, W.E.; Mitchell, R.L.; Millet, M.A. Techniques for the determination of pulp constituents by quantitiative paper chromatography. Tappi J. 1954, 37, 336–343. [Google Scholar]
- McKee, L.S.; Sunner, H.; Anasontzis, G.E.; Toriz, G.; Gatenholm, P.; Bulone, V.; Vilaplana, F.; Olsson, L. A GH115 α-glucuronidase from Schizophyllum commune contributes to the synergistic enzymatic deconstruction of softwood glucuronoarabinoxylan. Biotechnol. Biofuels 2016, 9, 2. [Google Scholar] [CrossRef]
- Buchert, J.; Johansson, L.-S.; Pere, J.; Campbell, J.M. Analysis of the Surface Chemistry of Linen and Cotton Fabrics. Text. Res. J. 2001, 71, 626–629. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved cellulose X-ray diffraction analysis using Fourier series modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J.; Miller, S.J. Effects of Two-Stage Dilute Acid Pretreatment on the Structure and Composition of Lignin and Cellulose in Loblolly Pine. BioEnergy Res. 2008, 1, 205–214. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Carbohydrate Reactions During High-Temperature Steam Treatment of Aspen Wood. Appl. Biochem. Biotechnol. 2005, 125, 175–188. [Google Scholar] [CrossRef]
- Sasaki, C.; Kiyokawa, A.; Asada, C.; Nakamura, Y. Glucose and Valuable Chemicals Production from Cotton Waste Using Hydrothermal Method. Waste Biomass Valorization 2019, 10, 599–607. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Po-Ju, L.; Wu, Y.-Q.; Ye, L.-Y.; Yang, D.-J.; Shieh, C.-J.; Lee, C.-K. Simultaneous Saccharification and Fermentation of Waste Textiles for Ethanol Production. BioResources 2014, 9, 2866–2875. [Google Scholar] [CrossRef]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; de Vries, J.G.; Heeres, H.J. Caprolactam from renewable resources: Catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef]
- Garg, S.; Jain, A. Fermentative production of 2,3-butanediol: A review. Bioresour. Technol. 1995, 51, 103–109. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Fakruddin, M.; Quayum, A.; Ahmed, M.M.; Choudhury, N. Analysis of key factors affecting ethanol production by saccharomyces cerevisiae IFST-072011. Biotechnology 2012, 11, 248–252. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Wolfaardt, F.J.; Leite Fernandes, L.G.; Cangussu Oliveira, S.K.; Duret, X.; Görgens, J.F.; Lavoie, J.-M. Recovery approaches for sulfuric acid from the concentrated acid hydrolysis of lignocellulosic feedstocks: A mini-review. Energy Convers. Manag. X 2021, 10, 100074. [Google Scholar] [CrossRef]
- Gregor, H.P.; Jeffries, T.W. Ethanolic fuels from renewable resources in the solar age. Ann. N. Y. Acad. Sci. 1979, 326, 273–287. [Google Scholar] [CrossRef]





| Study | Glucose Yield (%) | Glucose Concentration (G/L) | Solids Loading (Dry G Matter/G Solution) | Raw Material Used |
|---|---|---|---|---|
| Kong-Win Chang et al. [17] | 98 | - | 0.03 | Cellulose from steam-pretreated straw |
| Sasaki et al. without enzymes [36] | 29 | - | 0.03 | Cotton towels |
| Sasaki et al. with enzymes [36] | 78 | - | 0.02 | Cotton towels |
| Kuo et al. [37] | 80 | 45 | 0.08 | Waste cotton T-shirts |
| Our previous study [22] | 92 | 3 | 0.06 | Waste cotton bed linens |
| Present study | 72 | 109 | 0.74 | Waste cotton bed linens |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruuth, E.; Sanchis-Sebastiá, M.; Larsson, P.T.; Teleman, A.; Jiménez-Quero, A.; Delestig, S.; Sahlberg, V.; Salén, P.; Sanchez Ortiz, M.; Vadher, S.; et al. Reclaiming the Value of Cotton Waste Textiles: A New Improved Method to Recycle Cotton Waste Textiles via Acid Hydrolysis. Recycling 2022, 7, 57. https://doi.org/10.3390/recycling7040057
Ruuth E, Sanchis-Sebastiá M, Larsson PT, Teleman A, Jiménez-Quero A, Delestig S, Sahlberg V, Salén P, Sanchez Ortiz M, Vadher S, et al. Reclaiming the Value of Cotton Waste Textiles: A New Improved Method to Recycle Cotton Waste Textiles via Acid Hydrolysis. Recycling. 2022; 7(4):57. https://doi.org/10.3390/recycling7040057
Chicago/Turabian StyleRuuth, Edvin, Miguel Sanchis-Sebastiá, Per Tomas Larsson, Anita Teleman, Amparo Jiménez-Quero, Sara Delestig, Viktor Sahlberg, Patricia Salén, Marjorie Sanchez Ortiz, Simran Vadher, and et al. 2022. "Reclaiming the Value of Cotton Waste Textiles: A New Improved Method to Recycle Cotton Waste Textiles via Acid Hydrolysis" Recycling 7, no. 4: 57. https://doi.org/10.3390/recycling7040057
APA StyleRuuth, E., Sanchis-Sebastiá, M., Larsson, P. T., Teleman, A., Jiménez-Quero, A., Delestig, S., Sahlberg, V., Salén, P., Sanchez Ortiz, M., Vadher, S., & Wallberg, O. (2022). Reclaiming the Value of Cotton Waste Textiles: A New Improved Method to Recycle Cotton Waste Textiles via Acid Hydrolysis. Recycling, 7(4), 57. https://doi.org/10.3390/recycling7040057