Highlighting the Role of Archaea in Urban Mine Waste Exploitation and Valorisation
Abstract
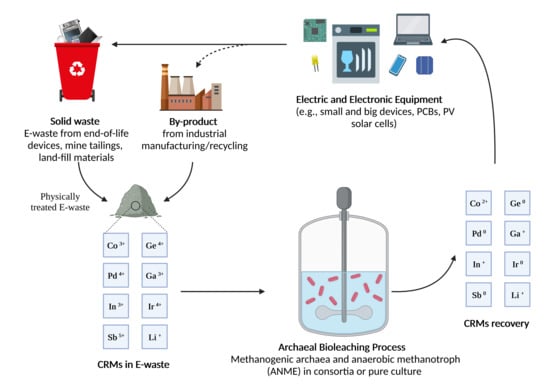
:1. Introduction
- (1)
- Large household machines (i.e., washing machines, refrigerators, dryers, air conditioners, dishwashers, etc.);
- (2)
- Small household machines (i.e., vacuum cleaners, microwaves, ventilation equipment, toasters, electric kettles, electric shavers, calculators, radio sets, video cameras, scales);
- (3)
- Information technology and communication (ICT) equipment (i.e., PCs, laptops, mobile phones, fax machines, printers, telephones, and photocopiers);
- (4)
- Consumer electronics (i.e., TV, VCR/DVD/CD players, hi-Fi sets, radios, train sets, coin slot machines);
- (5)
- Lighting Fluorescent lamps, high-intensity discharge lamps, and LED lamps;
- (6)
- Electrical and electronic tools (i.e., drills, electric saws, sewing machines, lawnmowers, large stationary tools);
- (7)
- Toys (i.e., electrical and electronic toys);
- (8)
- Leisure and sports equipment;
- (9)
- Medical devices (surveillance and control equipment, medical instruments and equipment);
- (10)
- Automatic dispensers.
2. Recycling E-Waste via Biological Route
2.1. Interactions between Archaea and Metals
2.2. Bioleaching among Archaea
2.2.1. Extremophile Archaeon
2.2.2. Methanogenic Archaea
Consortia
Pure Culture in H2/CO2
2.2.3. Adverse Interactions between Methanogens and Critical Metals
- Inhibition and toxicity: 50% inhibiting concentrations (IC50) for Cd, selenite, tellurite, and tellurate have been investigated on methanogenic consortia, with acetoclastic methanogens displaying higher IC50 (IC50Cd 8.6 mg/L, IC50selenite 24.1 mg/L, IC50tellurite 8.6 mg/L, IC50tellurate 10.2 mg/L) than those of hydrogenotrophic methanogens (IC50Cd 2.9 mg/L, IC50selenite 18 mg/L, IC50tellurite 8.6 mg/L, IC50tellurate 10.2 mg/L) [96]. Similarly, IC50 for Pd(II), Pt(II) and Pt(IV) for methanogens were reported at 2.7, 2.4 and 3.7 mg/L, respectively [85], whilst full inhibition of methanogenesis occurred at Pt(II) concentration higher than 5 mg/L [97];
- Competition between metal reduction and methanogenesis: Methanogenesis, both in anaerobic digestion and in the biomethanation process, requires electron donors to reduce CO2. When considering the bioleaching process, the addition of an external electron donor is often needed due to the competition for electrons that are redirected from methanogenesis to metal reduction [91]. In an anaerobic granular sludge, endogenous substrates can provide sufficient electron equivalents for the leaching of metals of interest, although reduction rates may increase with excess electrons, as reported by [87]. Among external electron donors to be added to methanogenic anaerobic sludge, ethanol is considered a safe and economical option. Fermentation of ethanol by the acetogenic community populating the granular sludge generates H2 that is then redirected to metal reduction. However,it should be mentioned that some studies have described the abiotic reduction of some metals through direct chemical reduction by H2 and formate [85,97,98].
2.2.4. Anaerobic Methanotrophic Archaea (ANME)
3. EU-Founded Projects Involving Bioleaching for CRMs Recovery
| Project Acronym | Funding Program | Project Goals | Project Budget | Duration | Ref. |
|---|---|---|---|---|---|
| Bioshale | Sixth Framework Programme (EU-70%) | Identification and development of innovative biotechnological processes for a safe, clean and viable exploitation of metal-rich black shale ores for metal production, and design of an innovative model of development of mining activities | €3,390,202 | 1 October 2004–30 September 2007 | [117,118] |
| BioMinE | Sixth Framework Programme (EU-65%) | Development, improvement and integration of bioleach processes for the recovery of metals from primary and secondary metal-bearing materials | €17,442,380 | 1 December 2004–31 October 2008 | [119] |
| ProMine | Seventh Framework Programme (EU-65%) | Development of new mineral-based nano-products and new technologies for strategic mineral supply, to stimulate the extractive industry to deliver new products to manufacturing industry | €17,232,739.10 | 1 May 2009–30 April 2013 | [120,121] |
| SysMetEx (ERASysAPP) | Seventh Framework Programme (EU-90%) | Investigation of biofilm formation on the surface of the world’s most abundant copper mineral, chalcopyrite, by acidophilic microorganisms interacting with the copper mineral and each other | €2,537,425 | 2015–2018 | [122,123,124,125,126] |
| BioMOre | Horizon 2020 (EU-100%) | Development of a novel base metal mining technology coupling in situ leaching and bioleaching technologies to deep deposits, in order to reduce environmental and social impacts and operating costs of mining techniques | €8,564,961.75 | 1 February 2015–31 July 2018 | [127] |
| BioFlex | EIT RawMaterials (EU co-funded) | Bringing together partners with infrastructure and expertise in biometallurgy including metals bioleaching from ores and waste, biosorption from liquid streams, bioprecipitation and bio-electrochemistry | NA | 1 January 2016–31 December 2018 | [128] |
| RUBICON | The European innovation partnership (EIP) on raw materials | Development of a novel biotechnical process for sustainable exploitation of laterites, polymetallic deep-sea nodules and weathered sulphide ore deposits in the EU. Definition of a downstream process for the specific recovery of the metal by-products cobalt and scandium, together with nickel and other solubilised metals (e.g., Cu, Zn, V, and Mn) | NA | 1 March 2016–1 March 2020 | [110] |
| BIOCriticalMetals (ERA-MIN) | Seventh Framework Programme (EU-95%) | Combining microorganisms having the potential to be used in the extraction of metals, with methods (bio and nano) to adsorb them to exploit potentially critical high-tech metals (W, In, Ga, Te, and Mo) tailings | €546,366 | 1 June 2016–31 December 2019 | [111,129,130] |
| BiotaWEE | LIFE Programme (EU-60%) | Recovering of valuable metals (mainly Cu, Ag and Au) from the non-metallic fraction of the Printed Circuit Boards (PCB) of different Waste Electric and Electronic Equipment (WEEE) by the application of an innovative more efficient 2-step bioleaching technology, combining aerobic and anaerobic treatment | €932,377 | 1 July 2018–31 July 2022 | [112] |
| BioLeach | EIT RawMaterials (EU co-funded) | Development and improvement of bioleaching technology for specific local deposits to obtain raw materials (RMs) appropriate for industrial utilization and broad the utilisation of local sources | NA | 1 April 2019–31 March 2022 | [131] |
| BIORECOVER | Horizon 2020 (EU-100%) | Research and development of a new sustainable and safe biotechnological process for the selective extraction of a wide range of Critical Raw Materials (CRMs) | €6,337,277.50 | 1 June 2019–31 May 2023 | [106,107,132] |
| RAWMINA | Horizon 2020 (EU-85%) | Implementation and standardisation of a continuous pilot process integrating novel bioleaching and nano-based materials for Sb, Co, Ge, and W selective recovery from Mine Waste (MW) from unexploited/underexploited metal-containing materials | €10,857,402.68 | 1 May 2021–31 October 2024 | [108,109] |
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. IPCC, 2022: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 2022nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Consolidated Text: Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE) (Recast) (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2012/19/2018-07-04 (accessed on 29 January 2023).
- Forti, V.; Baldé, C.P.; Kuehr, R. E-Waste Statistics: Guidelines on Classifications, Reporting and Indicators, 2nd ed.; United Nation University, Ed.; Tokio: Tokyo, Japan, 2018; ISBN 9789280890662. [Google Scholar]
- Priya, A.; Hait, S. Extraction of Metals from High Grade Waste Printed Circuit Board by Conventional and Hybrid Bioleaching Using Acidithiobacillus Ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Bizzo, W.A.; Figueiredo, R.A.; de Andrade, V.F. Characterization of Printed Circuit Boards for Metal and Energy Recovery after Milling and Mechanical Separation. Materials 2014, 7, 4555–4566. [Google Scholar] [CrossRef]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential; United Nations University: Bonn, Germany, 2020; ISBN 9789280891140. [Google Scholar]
- Huisman, J.; Leroy, P.; Tertre, F.; Söderman, M.L.; Chancerel, P.; Cassard, D.; Amund, N.; Wäger, P.; Kushnir, D.; Rotter, V.S.; et al. Prospecting Secondary Raw Materials in the Urban Mine and Mining Wastes (ProSUM)—Final Report; ProSUM Consortium: Brussels, Belgium, 2017. [Google Scholar]
- Baldé, C.P.; D’Angelo, E.; Luda, V.; Deubzer, O.; Kuehr, R. Kuehr Global Transboundary E-Waste Flows Monitor—2022; International Telecommunication Union: Bonn, Germany, 2022. [Google Scholar]
- Ghisellini, P.; Ncube, A.; Casazza, M.; Passaro, R. Toward Circular and Socially Just Urban Mining in Global Societies and Cities: Present State and Future Perspectives. Front. Sustain. Cities 2022, 4, 135. [Google Scholar] [CrossRef]
- Rizos, V.; Bryhn, J. Implementation of circular economy approaches in the electrical and electronic equipment (EEE) sector: Barriers, enablers and policy insights. J. Clean. Prod. 2022, 338. [Google Scholar] [CrossRef]
- Globalisation: How the EU’s Trade Policy Helps to Promote Human Rights. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20190612STO54309/globalisation-how-eu-trade-policy-helps-promote-human-rights (accessed on 2 February 2023).
- James, M. 2022 Final List of Critical Minerals. Available online: https://www.iea.org/policies/15271-final-list-of-critical-minerals-2022 (accessed on 2 February 2023).
- Energy Act of 2020 (Critical Minerals Provisions). Available online: https://www.iea.org/policies/16065-energy-act-of-2020-critical-minerals-provisions (accessed on 2 February 2023).
- Direzione generale del Mercato interno, dell’industria, dell’imprenditoria e delle P (Commissione Europea). Blengini, G.A., el Latunussa, C., Eynard, U., Torres De Matos, C., Wittmer, D., Georgitzikis, K., Pavel, C., Carrara, S., Mancini, L., et al., Eds.; Study on the EU’s List of Critical Raw Materials (2020); Final Report; Commissione Europea: Brussels, Belgium, 2020. [Google Scholar]
- Abdelbasir, S.M.; Hassan, S.S.M.; Kamel, A.H.; El-Nasr, R.S. Status of electronic waste recycling techniques: A review. Environ. Sci. Pollut. Res. 2018, 25, 16533–16547. [Google Scholar] [CrossRef] [PubMed]
- Velis, C.; Mavropoulos, A. Unsound waste management and public health: The neglected link? Waste Manag. Res. J. A Sustain. Circ. Econ. 2016, 34, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jia, S.; Ramakrishna, S. End-of-Life Photovoltaic Modules. Energies 2022, 15, 5113. [Google Scholar] [CrossRef]
- Nuss, P.; Eckelman, M.J. Life Cycle Assessment of Metals: A Scientific Synthesis. PLoS ONE 2014, 9, e101298. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S.; Agcasulu, I. A Review on Chemical versus Microbial Leaching of Electronic Wastes with Emphasis on Base Metals Dissolution. Minerals 2021, 11, 1255. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Wang, H.; Liu, L.; Wang, X. Pyrolysis Characteristics and Non-Isothermal Kinetics of Integrated Circuits. Materials 2022, 15, 4460. [Google Scholar] [CrossRef]
- Mirazimi, S.; Abbasalipour, Z.; Rashchi, F. Vanadium removal from LD converter slag using bacteria and fungi. J. Environ. Manag. 2015, 153, 144–151. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Fei, Y.; Cheng, Y. Novel Pathway for Vanadium(V) Bio-Detoxification by Gram-Positive Lactococcus raffinolactis. Environ. Sci. Technol. 2021, 55, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Willner, J.; Fornalczyk, A.; Gajda, B.; Saternus, M. Bioleaching of indium and tin from used LCD panels. Physicochem. Probl. Miner. Process. 2018, 54, 639–645. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Springer Science & Business Media: New York, NY, USA, 2012; Volume 101, pp. 1–30. [Google Scholar] [CrossRef]
- Ranawat, P.; Rawat, S. Metal-tolerant thermophiles: Metals as electron donors and acceptors, toxicity, tolerance and industrial applications. Environ. Sci. Pollut. Res. 2017, 25, 4105–4133. [Google Scholar] [CrossRef]
- Valls, M.; De Lorenzo, V. Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. A novel step-wise indirect bioleaching using biogenic ferric agent for enhancement recovery of valuable metals from waste light emitting diode (WLED). J. Hazard. Mater. 2019, 378, 120648. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Development and application of biotechnologies in the metal mining industry. Environ. Sci. Pollut. Res. 2013, 20, 7768–7776. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Castro, C.; Urbieta, M.; Cazón, J.P.; Donati, E. Metal biorecovery and bioremediation: Whether or not thermophilic are better than mesophilic microorganisms. Bioresour. Technol. 2019, 279, 317–326. [Google Scholar] [CrossRef]
- Sarkodie, E.K.; Jiang, L.; Li, K.; Yang, J.; Guo, Z.; Shi, J.; Deng, Y.; Liu, H.; Jiang, H.; Liang, Y.; et al. A review on the bioleaching of toxic metal(loid)s from contaminated soil: Insight into the mechanism of action and the role of influencing factors. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- van Wolferen, M.; Orell, A.; Albers, S.-V. Archaeal biofilm formation. Nat. Rev. Genet. 2018, 16, 699–713. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Mogharabi-Manzari, M.; Brandl, H. Bioleaching of metals from wastes and low-grade sources by HCN-forming microorganisms. Hydrometallurgy 2019, 191, 105228. [Google Scholar] [CrossRef]
- Magoda, K.; Mekuto, L. Biohydrometallurgical Recovery of Metals from Waste Electronic Equipment: Current Status and Pro-posed Process. Recycling 2022, 7, 67. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef] [PubMed]
- Krzmarzick, M.J.; Taylor, D.K.; Fu, X.; McCutchan, A.L. Diversity and niche of archaea in bioremediation. Hindawi 2018, 2018, 3194108. [Google Scholar] [CrossRef]
- Pfeifer, K.; Ergal, I.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.-M. Archaea Biotechnology. Biotechnol. Adv. 2020, 47, 107668. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef]
- Chakankar, M.; Su, C.H.; Hocheng, H. Leaching of metals from end-of-life solar cells. Environ. Sci. Pollut. Res. 2018, 26, 29524–29531. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Feasibility of Bioleaching of Selected Metals from Electronic Waste by Acidiphilium acidophilum. Waste Biomass- Valorization 2017, 9, 871–877. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Comparative assessment of metallurgical recovery of metals from electronic waste with special emphasis on bioleaching. Environ. Sci. Pollut. Res. 2017, 24, 6989–7008. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A novel eco-friendly hybrid approach for recovery and reuse of copper from electronic waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Liu, Y.; Whitman, W.B. Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea. Ann. New York Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Basso, O.; Lascourreges, J.-F.; Le Borgne, F.; Le Goff, C.; Magot, M. Characterization by culture and molecular analysis of the microbial diversity of a deep subsurface gas storage aquifer. Res. Microbiol. 2009, 160, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bini, E. Archaeal transformation of metals in the environment. FEMS Microbiol. Ecol. 2010, 73, 1–16. [Google Scholar] [CrossRef]
- Oren, A. Taxonomy of halophilic Archaea: Current status and future challenges. Extremophiles 2014, 18, 825–834. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Sar, P.; Kazy, S.K.; Paul, D.; Sarkar, A. Metal Bioremediation by Thermophilic Microorganisms. In Thermophilic Microbes in Environmental and Industrial Biotechnology: Biotechnology of Thermophiles; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9789400758995. [Google Scholar]
- Dupont, C.L.; Yang, S.; Palenik, B.; Bourne, P.E. Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc. Natl. Acad. Sci. USA 2006, 103, 17822–17827. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Elmi, S.; Rosato, A. Non-Heme Iron Through the Three Domains of Life. Proteins: Struct. Funct. Bioinform. 2007, 67, 317–324. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Occurrence of Copper Proteins through the Three Domains of Life: A Bioinformatic Approach. J. Proteome Res. 2007, 7, 209–216. [Google Scholar] [CrossRef]
- Lyu, Z.; Chou, C.-W.; Shi, H.; Wang, L.; Ghebreab, R.; Phillips, D.; Yan, Y.; Duin, E.C.; Whitman, W.B. Assembly of Methyl Coenzyme M Reductase in the Methanogenic Archaeon Methanococcus maripaludis. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef]
- Tsurumaru, H.; Ito, N.; Mori, K.; Wakai, S.; Uchiyama, T.; Iino, T.; Hosoyama, A.; Ataku, H.; Nishijima, K.; Mise, M.; et al. An extracellular [NiFe] hydrogenase mediating iron corrosion is encoded in a genetically unstable genomic island in Methanococcus maripaludis. Sci. Rep. 2018, 8, 15149. [Google Scholar] [CrossRef]
- Rodionov, D.A.; Hebbeln, P.; Gelfand, M.; Eitinger, T. Comparative and Functional Genomic Analysis of Prokaryotic Nickel and Cobalt Uptake Transporters: Evidence for a Novel Group of ATP-Binding Cassette Transporters. J. Bacteriol. 2006, 188, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; A Rodionov, D.; Gelfand, M.S.; Gladyshev, V.N. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genom. 2009, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gladyshev, V.N. Molybdoproteomes and Evolution of Molybdenum Utilization. J. Mol. Biol. 2008, 379, 881–899. [Google Scholar] [CrossRef]
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.A.; Vasile, N.S.; Pirri, C.F.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064. [Google Scholar] [CrossRef]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Schattauer, A.; Abdoun, E.; Weiland, P.; Plöchl, M.; Heiermann, M. Abundance of trace elements in demonstration biogas plants. Biosyst. Eng. 2010, 108, 57–65. [Google Scholar] [CrossRef]
- Wintsche, B.; Jehmlich, N.; Popp, D.; Harms, H.; Kleinsteuber, S. Metabolic Adaptation of Methanogens in Anaerobic Digesters Upon Trace Element Limitation. Front. Microbiol. 2018, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Giuliano, M.; De Rosa, M. Perspectives on biotechnological applications of archaea. Archaea 2002, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.T.; A Counts, J.; Nguyen, D.M.N.; Wu, C.-H.; Zeldes, B.M.; Crosby, J.R.; Conway, J.M.; Otten, J.K.; Lipscomb, G.L.; Schut, G.J.; et al. Biotechnology of extremely thermophilic archaea. FEMS Microbiol. Rev. 2018, 42, 543–578. [Google Scholar] [CrossRef]
- Hedlund, B.P.; Zhang, C.; Wang, F.; Rinke, C.; Martin, W.F. Editorial: Ecology, Metabolism and Evolution of Archaea-Perspectives from Proceedings of the International Workshop on Geo-Omics of Archaea. Front. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Siezen, R.J.; Wilson, G. Bioleaching genomics. Microb. Biotechnol. 2009, 2, 297–303. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Yakimov, M.M.; Lünsdorf, H.; Ferrer, M.; Nimtz, M.; Timmis, K.N.; Wray, V.; Tindall, B.J.; Golyshin, P.N. Acidiplasma aeolicum gen. nov., sp. nov., a euryarchaeon of the family Ferroplasmaceae isolated from a hydrothermal pool, and transfer of Ferroplasma cupricumulans to Acidiplasma cupricumulans comb. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2815–2823. [Google Scholar] [CrossRef]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Microbiol. 1972, 84, 54–68. [Google Scholar] [CrossRef]
- Segerer, A.H.; Trincone, A.; Gahrtz, M.; Stetter, K.O. Stygiolobus azoricus gen. nov., sp. nov. Represents a Novel Genus of Anaerobic, Extremely Thermoacidophilic Archaebacteria of the Order Sulfolobales. Int. J. Syst. Evol. Microbiol. 1991, 41, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Fütterer, O.; Angelov, A.; Liesegang, H.; Gottschalk, G.; Schleper, C.; Schepers, B.; Dock, C.; Antranikian, G.; Liebl, W. Genome sequence of Picrophilus torridus and its implications for life around pH 0. Proc. Natl. Acad. Sci. 2004, 101, 9091–9096. [Google Scholar] [CrossRef]
- Itoh, T.; Yoshikawa, N.; Takashina, T. Thermogymnomonas acidicola gen. nov., sp. nov., a novel thermoacidophilic, cell wall-less archaeon in the order Thermoplasmatales, isolated from a solfataric soil in Hakone, Japan. Int. J. Syst. Evol. Microbiol. 2007, 57, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Segerer, A.; Langworthy, T.A.; Stetter, K.O. Thermoplasma acidophilum and Thermoplasma volcanium sp. nov. from Solfatara Fields. Syst. Appl. Microbiol. 1988, 10, 161–171. [Google Scholar] [CrossRef]
- Sehlin, H.M.; Lindstrã¶m, E.B. Oxidation and reduction of arsenic by Sulfolobus acidocaldarius strain BC. FEMS Microbiol. Lett. 1992, 93, 87–92. [Google Scholar] [CrossRef]
- Lebrun, E.; Brugna, M.; Baymann, F.; Muller, D.; Lièvremont, D.; Lett, M.-C.; Nitschke, W. Arsenite Oxidase, an Ancient Bioenergetic Enzyme. Mol. Biol. Evol. 2003, 20, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Heinrich-Salmeron, A.; Cordi, A.; Brochier-Armanet, C.; Halter, D.; Pagnout, C.; Abbaszadeh-Fard, E.; Montaut, D.; Seby, F.; Bertin, P.N.; Bauda, P.; et al. Unsuspected Diversity of Arsenite-Oxidizing Bacteria as Revealed by Widespread Distribution of the aoxB Gene in Prokaryotes. Appl. Environ. Microbiol. 2011, 77, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, O.F.; Rasuk, M.C.; Soria, M.N.; Contreras, M.; Farías, M.E. Haloarchaea from the Andean Puna: Biological Role in the Energy Metabolism of Arsenic. Microb. Ecol. 2018, 76, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Boyd, E.; Crane, S.; Lu-Irving, P.; Krabbenhoft, D.; King, S.; Dighton, J.; Geesey, G.; Barkay, T. Environmental Conditions Constrain the Distribution and Diversity of Archaeal merA in Yellowstone National Park, Wyoming, U.S.A. Microb. Ecol. 2011, 62, 739–752. [Google Scholar] [CrossRef]
- Schelert, J.; Dixit, V.; Hoang, V.; Simbahan, J.; Drozda, M.; Blum, P. Occurrence and Characterization of Mercury Resistance in the Hyperthermophilic Archaeon Sulfolobus solfataricus by Use of Gene Disruption. J. Bacteriol. 2004, 186, 427–437. [Google Scholar] [CrossRef]
- Al-Mailem, D.M.; Al-Awadhi, H.; Sorkhoh, N.A.; Eliyas, M.; Radwan, S.S. Mercury resistance and volatilization by oil utilizing haloarchaea under hypersaline conditions. Extremophiles 2010, 15, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.R.; Szymanowski, J.E.S.; Fein, J.B.; A Bunker, B. An x-ray absorption spectroscopy study of Cd binding onto a halophilic archaeon. J. Phys. Conf. Ser. 2016, 712, 12079. [Google Scholar] [CrossRef]
- Das, D.; Salgaonkar, B.B.; Mani, K.; Braganca, J.M. Cadmium resistance in extremely halophilic archaeon Haloferax strain BBK2. Chemosphere 2014, 112, 385–392. [Google Scholar] [CrossRef]
- Orange, F.; Westall, F.; Disnar, J.-R.; Prieur, D.; Bienvenu, N.; LE Romancer, M.; Défarge, C. Experimental silicification of the extremophilic Archaea Pyrococcus abyssi and Methanocaldococcus jannaschii: Applications in the search for evidence of life in early Earth and extraterrestrial rocks. Geobiology 2009, 7, 403–418. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Field, J.A.; Otero-Gonzalez, L.; Razo-Flores, E.; Cervantes, F.J.; Sierra-Alvarez, R. Recovery of palladium(II) by methanogenic granular sludge. Chemosphere 2016, 144, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K. Chemical and Microbial Processes for Rhodium Recovery in the Graduate College. Ph.D. Thesis, The University of Arizona, Tucson, Arizona, 2022. [Google Scholar]
- Ramos-Ruiz, A.; Field, J.A.; Wilkening, J.V.; Sierra-Alvarez, R. Recovery of Elemental Tellurium Nanoparticles by the Reduction of Tellurium Oxyanions in a Methanogenic Microbial Consortium. Environ. Sci. Technol. 2016, 50, 1492–1500. [Google Scholar] [CrossRef]
- Calvert, G.; Kaksonen, A.H.; Cheng, K.Y.; Van Yken, J.; Chang, B.; Boxall, N.J. Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide. Minerals 2019, 9, 563. [Google Scholar] [CrossRef]
- Kim, B.K.; Pihl, T.D.; Reeve, J.N.; Daniels, L. Purification of the copper response extracellular proteins secreted by the copper-resistant methanogen Methanobacterium bryantii BKYH and cloning, sequencing, and transcription of the gene encoding these proteins. J. Bacteriol. 1995, 177, 7178–7185. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; Lange, M.; Ahring, B.K.; De Macario, E.C. Stress Genes and Proteins in the Archaea. Microbiol. Mol. Biol. Rev. 1999, 63, 923–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, H.; Zhao, L.; McCarrick, R.; Agrawal, A. Microbial reduction and precipitation of vanadium by mesophilic and thermophilic methanogens. Chem. Geol. 2014, 370, 29–39. [Google Scholar] [CrossRef]
- Singh, R.; Dong, H.; Liu, D.; Marts, A.R.; Tierney, D.L.; Almquist, C.B. [Cobalt(III)–EDTA]−reduction by thermophilic methanogen Methanothermobacter thermautotrophicus. Chem. Geol. 2015, 411, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dong, H.; Liu, D.; Zhao, L.; Marts, A.R.; Farquhar, E.; Tierney, D.L.; Almquist, C.B.; Briggs, B.R. Reduction of Hexavalent Chromium by the Thermophilic Methanogen Methanothermobacter thermautotrophicus. Geochim. Et Cosmochim. Acta 2015, 148, 442–456. [Google Scholar] [CrossRef]
- E Holmes, D.; Orelana, R.; Giloteaux, L.; Wang, L.-Y.; Shrestha, P.; Williams, K.; Lovley, D.R.; Rotaru, A.-E. Potential for Methanosarcina to Contribute to Uranium Reduction during Acetate-Promoted Groundwater Bioremediation. Microb. Ecol. 2018, 76, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Orange, F.; Disnar, J.-R.; Westall, F.; Prieur, D.; Baillif, P. Metal cation binding by the hyperthermophilic microorganism, Archaea Methanocaldococcus Jannaschii, and its effects on silicification. Palaeontology 2011, 54, 953–964. [Google Scholar] [CrossRef]
- Ramos-Ruiz, A.; Zeng, C.; Sierra-Alvarez, R.; Teixeira, L.H.; Field, J.A. Microbial Toxicity of Ionic Species Leached from the II-VI Semiconductor Materials, Cadmium Telluride (CdTe) and Cadmium Selenide (CdSe). Chemosphere 2016, 162, 131–138. [Google Scholar] [CrossRef]
- Simon-Pascual, A.; Sierra-Alvarez, R.; Field, J.A. Platinum(II) reduction to platinum nanoparticles in anaerobic sludge. J. Chem. Technol. Biotechnol. 2018, 94, 468–474. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Jiang, W.; Finster, K.; Skrydstrup, T.; Meyer, R.L. Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnol. Bioeng. 2012, 109, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Knittel, K.; Boetius, A.; Offre, P.; Spang, A.; Schleper, C.; Strous, M.; Jetten, M.S.; Pearson, A.; Ingalls, A.E.; Boucher, Y.; et al. Anaerobic Oxidation of Methane: Progress with an Unknown Process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Dong, Q.-Y.; Rittmann, B.E.; Zhao, H.-P. Bioreduction of Antimonate by Anaerobic Methane Oxidation in a Membrane Biofilm Batch Reactor. Environ. Sci. Technol. 2018, 52, 8693–8700. [Google Scholar] [CrossRef]
- Goyal, N.; Zhou, Z.; Karimi, I.A. Metabolic processes of Methanococcus maripaludis and potential applications. Microb. Cell Factories 2016, 15, 1–19. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Zuo, K.; He, C.; Dai, Y.; Ren, Z.J. Microbial vanadate and nitrate reductions coupled with anaerobic methane oxidation in groundwater. J. Hazard. Mater. 2019, 382, 121228. [Google Scholar] [CrossRef]
- Luo, J.-H.; Wu, M.; Yuan, Z.; Guo, J. Biological Bromate Reduction Driven by Methane in a Membrane Biofilm Reactor. Environ. Sci. Technol. Lett. 2017, 4, 562–566. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, R.; Hou, J.; Lv, Z.; Li, L.; Hu, Y.; Huang, H.; Wang, F. The late Archaean to early Proterozoic origin and evolution of anaerobic methane-oxidizing archaea. Mlife 2022, 1, 96–100. [Google Scholar] [CrossRef]
- Leu, A.O.; Cai, C.; McIlroy, S.J.; Southam, G.; Orphan, V.J.; Yuan, Z.; Hu, S.; Tyson, G.W. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J. 2020, 14, 1030–1041. [Google Scholar] [CrossRef]
- Development of an Innovative Sustainable Strategy for Selective Biorecover of Critical Raw Materials from Primary and Sec-ondary Sources. Available online: https://cordis.europa.eu/project/id/821096 (accessed on 31 December 2022).
- BIORECOVER. Raw Materials. Sustainable. Available online: https://biorecover.eu/ (accessed on 31 December 2022).
- Integrated Innovative Pilot System for Critical Raw Materials Recovery from Mines Wastes in a Circular Economy Context. Available online: https://cordis.europa.eu/project/id/958252 (accessed on 31 December 2022).
- RAWMINA. An Integrated, Innovative Pilot System for Recovery of Critical Raw Materials from Mine Waste. Available online: https://rawmina.eu/ (accessed on 31 December 2022).
- Reductive Bioleaching for Extracting By-Products from Primary and Secondary Resources. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/eip/raw-materials-commitment/reductive-bioleaching-extracting-products-primary-and-secondary-resources_en (accessed on 31 December 2022).
- The Third ERA-MIN Joint Call (2015). Available online: https://www.era-learn.eu/network-information/networks/era-min/the-third-era-min-joint-call-2015/recognition-of-microbial-functional-communities-and-assessment-of-the-mineralizing-potential-bioleaching-for-high-tech-critical-metals (accessed on 31 December 2022).
- BiotaWee. Available online: https://www.biotawee.com/en/home (accessed on 31 December 2022).
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B, applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- N2s. Available online: https://www.n2s.co.uk/ (accessed on 31 December 2022).
- Biota Tec. Available online: https://biotatec.com/ (accessed on 31 December 2022).
- Ekolive. Bioleaching. Bioremediation. Biostimulants. Available online: https://ekolive.eu/ (accessed on 31 December 2022).
- Search for a Sustainable Way of Exploiting Black Shale Ores Using Biotechnologies. Available online: https://cordis.europa.eu/project/id/505710 (accessed on 31 December 2022).
- D’Hugues, P.; Norris, P.; Hallberg, K.; Sánchez, F.; Langwaldt, J.; Grotowski, A.; Chmielewski, T.; Groudev, S. Bioshale FP6 European project: Exploiting black shale ores using biotechnologies? Miner. Eng. 2008, 21, 111–120. [Google Scholar] [CrossRef]
- Biotechnology for Metal Bearing Materials in Europe. Available online: https://cordis.europa.eu/project/id/500329 (accessed on 31 December 2022).
- ProMine – Nano-Particle Products from New Mineral Resources in Europe. Available online: https://www.gtk.fi/en/research-project/promine-nano-particle-products-from-new-mineral-resources-in-europe/ (accessed on 31 December 2022).
- Nano-Particle Products from New Mineral Resources in Europe. Available online: https://cordis.europa.eu/project/id/228559 (accessed on 31 December 2022).
- Systems.Ch. Available online: http://www.systemsx.ch/projects/international-projects/sysmetex/ (accessed on 31 December 2022).
- Buetti-Dinh, A.; Herold, M.; Christel, S.; El Hajjami, M.; Bellenberg, S.; Ilie, O.; Wilmes, P.; Poetsch, A.; Sand, W.; Vera, M.; et al. Systems biology of acidophile biofilms for efficient metal extraction. Sci. Data 2020, 7, 215. [Google Scholar] [CrossRef]
- Christel, S.; Herold, M.; Bellenberg, S.; El Hajjami, M.; Buetti-Dinh, A.; Pivkin, I.V.; Sand, W.; Wilmes, P.; Poetsch, A.; Dopson, M. Multi-omics Reveals the Lifestyle of the Acidophilic, Mineral-Oxidizing Model Species Leptospirillum ferriphilum T. Appl. Environ. Microbiol. 2018, 84, e02091-17. [Google Scholar] [CrossRef]
- Christel, S.; Herold, M.; Bellenberg, S.; Buetti-Dinh, A.; El Hajjami, M.; Pivkin, I.V.; Sand, W.; Wilmes, P.; Poetsch, A.; Vera, M.; et al. Weak Iron Oxidation by Sulfobacillus thermosulfidooxidans Maintains a Favorable Redox Potential for Chalcopyrite Bioleaching. Front. Microbiol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Bellenberg, S.; Buetti-Dinh, A.; Galli, V.; Ilie, O.; Herold, M.; Christel, S.; Boretska, M.; Pivkin, I.V.; Wilmes, P.; Sand, W.; et al. Automated Microscopic Analysis of Metal Sulfide Colonization by Acidophilic Microorganisms. Appl. Environ. Microbiol. 2018, 84, e01835-18. [Google Scholar] [CrossRef] [PubMed]
- New Mining Concept for Extracting Metals from Deep Ore Deposits Using Biotechnology. Available online: https://cordis.europa.eu/project/id/642456 (accessed on 31 December 2022).
- Bioflex. Available online: https://eitrawmaterials.eu/project/bioflex/ (accessed on 31 December 2022).
- European Research Area—Network on the Industrial Handling of Raw Materials for European Industries. Available online: https://cordis.europa.eu/project/id/291870 (accessed on 31 December 2022).
- INMR. Available online: https://imnr.ro/wp/wp-content/uploads/workshop-III.pdf (accessed on 31 December 2022).
- BioLeach: Innovative Bio-Treatment of RM. Available online: https://eitrawmaterials.eu/project/bioleach/ (accessed on 31 December 2022).
- Alcasabas, A.; Massingberd-Mundy, F.; Breeze, B.; Pérez, M.R.; García, C.M. BIORECOVER—New Bio-based Technologies for Recapture of Critical Raw Materials. Johns. Matthey Technol. Rev. 2020, 65, 151–157. [Google Scholar] [CrossRef]


| Metal | Microorganism | Function in Archaea | References |
|---|---|---|---|
| Fe- | Halobacterium spp., Methanosarcina spp., Methanobacterium spp., Sulfolobus spp., Thermoplasma spp., Ferroplasma spp., Pyrobaculum spp. | Fe (II)oxidation, Fe (III) reduction, Fe4S4-ferredoxin, Fe4S4 cluster for S- adenosylmethionine cleavage, Ni-Fe hydrogenase | [48,52,54,55,56] |
| Zn- | n.s. | “Small proteins” class genes (Zn finger motifs and Really Interesting gene (RING)) | [52] |
| Co- | Methanosarcina spp., Sulfolobus solfataricus, Thermoplasma acidophilum | Found in co-enzyme B12 structure, Ni/Co uptake system | [57,58] |
| Ni- | Sulfolobus spp., Halobacter spp., Methanococcus spp. | Enzymatic co-factor for different enzymes:Ni-Fe hydrogenase, CO de-hydrogenase, methyl-CoM reductase, urease | [56,57,58] |
| Cu- | Halobacterium spp., Methanosarcina spp., Methanobacterium spp. | Copper-binding proteins, N2O reductase | [54] |
| Mo- | Sulfolobales spp., Halobacteriales spp., Methanosarcinales spp., Methanococcales spp., Methanomicrobiales spp. | Molybdenum co-factor (Moco) involved in W utilization | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Azim, A.; Bellini, R.; Vizzarro, A.; Bassani, I.; Pirri, C.F.; Menin, B. Highlighting the Role of Archaea in Urban Mine Waste Exploitation and Valorisation. Recycling 2023, 8, 20. https://doi.org/10.3390/recycling8010020
Abdel Azim A, Bellini R, Vizzarro A, Bassani I, Pirri CF, Menin B. Highlighting the Role of Archaea in Urban Mine Waste Exploitation and Valorisation. Recycling. 2023; 8(1):20. https://doi.org/10.3390/recycling8010020
Chicago/Turabian StyleAbdel Azim, Annalisa, Ruggero Bellini, Arianna Vizzarro, Ilaria Bassani, Candido Fabrizio Pirri, and Barbara Menin. 2023. "Highlighting the Role of Archaea in Urban Mine Waste Exploitation and Valorisation" Recycling 8, no. 1: 20. https://doi.org/10.3390/recycling8010020
APA StyleAbdel Azim, A., Bellini, R., Vizzarro, A., Bassani, I., Pirri, C. F., & Menin, B. (2023). Highlighting the Role of Archaea in Urban Mine Waste Exploitation and Valorisation. Recycling, 8(1), 20. https://doi.org/10.3390/recycling8010020