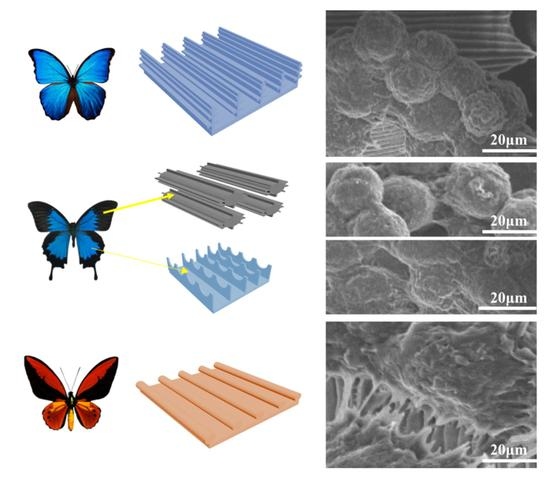
Hepatocyte Aggregate Formation on Chitin-Based Anisotropic Microstructures of Butterfly Wings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Methods
2.3.1. Treatment of Butterfly Wings
2.3.2. Cell Culture
2.3.3. Methylthiazolyldiphenyl-Tetrazolium Bromide Viability Assay
2.3.4. Fluorescent Viability Staining
2.3.5. Scanning Electron Microscopy
2.3.6. Assessment of Hepatic Function
2.3.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolun-Cholewa, M.; Langer, K.; Szymanowski, K.; Glodek, A.; Jankowska, A.; Warchol, W.; Langer, J. An efficient 3D cell culture method on biomimetic nanostructured grids. PLoS ONE 2013, 8, e72936. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, D.; Han, S. 3D printing of scaffold for cells delivery: Advances in skin tissue engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef]
- Park, S.; Park, K. Engineered polymeric hydrogels for 3D tissue models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Q.; Chu, X.H.; Huang, N.P.; Leach, M.K.; Wang, G.; Wang, Y.C.; Ding, Y.T.; Gu, Z.Z. Rat hepatocyte aggregate formation on discrete aligned nanofibers of type-I collagen-coated poly(l-lactic acid). Biomaterials 2010, 31, 3604–3612. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, X.; Liu, Y.; Zhang, L. Enhancing the compatibility, hydrophilicity and mechanical properties of polysulfone ultrafiltration membranes with lignocellulose nanofibrils. Polymers 2016, 8, 349. [Google Scholar] [CrossRef]
- Curcio, E.; Salerno, S.; Barbieri, G.; De Bartolo, L.; Drioli, E.; Bader, A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 2007, 28, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, W.; Jiang, X. Precise control of cell adhesion by combination of surface chemistry and soft lithography. Adv. Healthc. Mater. 2013, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Arazi, N.; Domb, A.J.; Katzhendler, J. New biocompatible polyesters derived from α-amino acids: hydrolytic degradation behavior. Polymers 2010, 2, 418–439. [Google Scholar] [CrossRef]
- Siddique, R.H.; Faisal, A.; Hünig, R.; Bartels, C.; Wacker, I.; Lemmer, U.; Hölscher, H. Utilizing laser interference lithography to fabricate hierarchical optical active nanostructures inspired by the blue Morpho butterfly. SPIE Proc. 2014, 9187, 91870E-1. [Google Scholar]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Han, D.; Cheung, K.C. Biodegradable cell-seeded nanofiber scaffolds for neural repair. Polymers 2011, 3, 1684–1733. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nano 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Sun, J.; Ding, J. Critical areas of cell adhesion on micropatterned surfaces. Biomaterials 2011, 32, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, K.; Li, Z.; Yan, C.; Ding, J. Adhesion, proliferation, and differentiation of mesenchymal stem cells on RGD nanopatterns of varied nanospacings. Organogenesis 2013, 9, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Szulgit, G.; Del Rio, J.A.; Barlow, C.; Bhatia, S.N. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology 2004, 40, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Michiardi, A.; Navarro, M.; Lacroix, D.; Planell, J.A. Nanotechnology in regenerative medicine: The materials side. Trends Biotechnol. 2008, 26, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.; O’Brien, F.; Cryan, S.-A. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.; Santo, V.; Rodrigues, M.; Gomes, M. Magnetically-responsive hydrogels for modulation of chondrogenic commitment of human adipose-derived stem cells. Polymers 2016, 8, 28. [Google Scholar] [CrossRef]
- Mhd Haniffa, M.; Ching, Y.; Abdullah, L.; Poh, S.; Chuah, C. Review of bionanocomposite coating films and their applications. Polymers 2016, 8, 246. [Google Scholar] [CrossRef]
- Gerecht, S.; Bettinger, C.J.; Zhang, Z.; Borenstein, J.T.; Vunjak-Novakovic, G.; Langer, R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials 2007, 28, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, Y.; Wang, H.; Ye, B.; Shang, L.; Zhao, Y.; Gu, Z. Multifunctional inverse opal particles for drug delivery and monitoring. Nanoscale 2015, 7, 10590–10594. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Hernandez, R.M.; Pedraz, J.L.; Orive, G. Novel advances in the design of three-dimensional bio-scaffolds to control cell fate: Translation from 2D to 3D. Trends Biotechnol. 2012, 30, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Udenni Gunathilake, T.; Ching, Y.; Ching, K.; Chuah, C.; Abdullah, L. Biomedical and microbiological applications of bio-based porous materials: A review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.H.; Shi, X.L.; Feng, Z.Q.; Gu, Z.Z.; Ding, Y.T. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol. Lett. 2009, 31, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wenhong, P.; Shenmin, Z.; Di, Z. Bio-inspired fabrication of stimuli-responsive photonic crystals with hierarchical structures and their applications. Nanotechnology 2016, 27, 122001. [Google Scholar]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, F.; Cheng, Y.; Ding, H.; Zhao, Y.; Gu, Z. Hybrid inverse opals for regulating cell adhesion and orientation. Nanoscale 2014, 6, 10650–10656. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Balikov, D.; Yang, J.; Kim, K.; Park, H.; Kim, J.; Kwon, I.; Bellan, L.; Sung, H.-J. Cationic nanocylinders promote angiogenic activities of endothelial cells. Polymers 2016, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Lizu, M.; Stewart, M.; Zygula, K.; Lu, Y.; Chauhan, R.; Yan, X.; Guo, Z.; Wujcik, E.; Wei, S. Multifunctional nanofibers towards active biomedical therapeutics. Polymers 2015, 7, 186–219. [Google Scholar] [CrossRef]
- Iwasaki, N.; Kasahara, Y.; Yamane, S.; Igarashi, T.; Minami, A.; Nisimura, S.-I. Chitosan-based hyaluronic acid hybrid polymer fibers as a scaffold biomaterial for cartilage tissue engineering. Polymers 2011, 3, 100–113. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Chu, X.; Huang, N.P.; Wang, T.; Wang, Y.; Shi, X.; Ding, Y.; Gu, Z.Z. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009, 30, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lu, W.; Ren, X.; Liu, W.; Guo, M.; Ullah, I.; Zhang, W. Electrospun poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) nanofibrous scaffolds for tissue engineering. Polymers 2016, 8, 13. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.; Liu, Y.; Liu, B.; Zhao, X. The 3-D culture and in vivogrowth of the human hepatocellular carcinoma cell line HepG2 in a self-assembling peptide nanofiber scaffold. J. Nanomater. 2010, 2010, 437219. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Gu, H.; Zhu, C.; Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 2012, 41, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Elbaz, A.; Lu, J.; Gao, B.; Zheng, F.; Mu, Z.; Zhao, Y.; Gu, Z. Chitin-based anisotropic nanostructures of butterfly wings for regulating cells orientation. Polymers 2017, 9, 386. [Google Scholar] [CrossRef]
- Mu, Z.; Zhao, X.; Xie, Z.; Zhao, Y.; Zhong, Q.; Bo, L.; Gu, Z. In situ synthesis of gold nanoparticles (AuNPs) in butterfly wings for surface enhanced Raman spectroscopy (SERS). J. Mater. Chem. B 2013, 1, 1607–1613. [Google Scholar] [CrossRef]
- Mekhail, M.; Tabrizian, M. Injectable chitosan-based scaffolds in regenerative medicine and their clinical translatability. Adv. Healthc. Mater. 2014, 3, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, S.; Jung, Y. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 2017, 9, 348. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P.; Zhu, C.; Elbaz, A.; Gu, Z.Z. Photonic crystal for gas sensing. J. Mater. Chem. C 2013, 1, 6087–6098. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbaz, A.; Gao, B.; He, Z.; Gu, Z. Hepatocyte Aggregate Formation on Chitin-Based Anisotropic Microstructures of Butterfly Wings. Biomimetics 2018, 3, 2. https://doi.org/10.3390/biomimetics3010002
Elbaz A, Gao B, He Z, Gu Z. Hepatocyte Aggregate Formation on Chitin-Based Anisotropic Microstructures of Butterfly Wings. Biomimetics. 2018; 3(1):2. https://doi.org/10.3390/biomimetics3010002
Chicago/Turabian StyleElbaz, Abdelrahman, Bingbing Gao, Zhenzhu He, and Zhongze Gu. 2018. "Hepatocyte Aggregate Formation on Chitin-Based Anisotropic Microstructures of Butterfly Wings" Biomimetics 3, no. 1: 2. https://doi.org/10.3390/biomimetics3010002
APA StyleElbaz, A., Gao, B., He, Z., & Gu, Z. (2018). Hepatocyte Aggregate Formation on Chitin-Based Anisotropic Microstructures of Butterfly Wings. Biomimetics, 3(1), 2. https://doi.org/10.3390/biomimetics3010002