Assessment of Optimal Conditions for Marine Invertebrate Cell-Mediated Mineralization of Organic Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collagen Preparation
2.2. Collagen Mineralization
2.3. pH Analysis
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopic (EDS) Analysis
2.5. X-ray Diffraction (XRD) Analysis
3. Results
3.1. Mineralization at Room Temperature
3.2. Mineralization at 37 °C
3.3. Mineralization at 30 °C
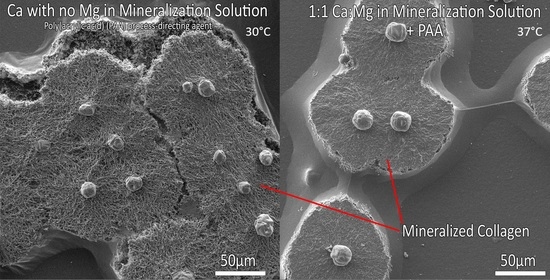
3.4. Influence of Calcium:Magnesium Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef] [Green Version]
- Launey, M.E.; Ritchie, R.O. On the fracture toughness of advanced materials. Adv. Mater. 2009, 21, 2103–2110. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef]
- Kul’chin, Y.N.; Bagaev, S.N.; Bukin, O.A.; Voznesenskiǐ, S.S.; Drozdov, A.L.; Zinin, Y.A.; Nagornyǐ, I.G.; Pestryakov, E.V.; Trunov, V.I. Photonic crystals based on natural oceanic biominerals. Tech. Phys. Lett. 2008, 34, 633–635. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Peres, B.U.; Carvalho, R.M.; MacLachlan, M.J. Photonic Hydrogels from Chiral Nematic Mesoporous Chitosan Nanofibril Assemblies. Adv. Funct. Mater. 2016, 26, 2875–2881. [Google Scholar] [CrossRef]
- Prozorov, T.; Bazylinski, D.A.; Mallapragada, S.K.; Prozorov, R. Novel magnetic nanomaterials inspired by magnetotactic bacteria: Topical review. Mater. Sci. Eng. R Rep. 2013, 74, 133–172. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W.; Lowenstam, H.A. Organic Matrix in Calcified Exoskeletons. Biominer. Biol. Met. Accumul. 1983, 205–224. [Google Scholar] [CrossRef]
- Landis, W.J.; Silver, F.H. Mineral Deposition in the Extracellular Matrices of Vertebrate Tissues: Identification of Possible Apatite Nucleation Sites on Type I Collagen. Cells Tissues Organs 2009, 189, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.F.; Nudelman, F.; Eren, E.D.; Wirix, M.J.M.; Cantaert, B.; Nijhuis, W.H.; Hermida-Merino, D.; Portale, G.; Bomans, P.H.H.; Ottmann, C.; et al. Intermolecular channels direct crystal orientation in mineralized collagen. Nat. Commun. 2020, 11, 5068. [Google Scholar] [CrossRef]
- Bin San Chan, V.; Johnstone, M.B.; Wheeler, A.P.; Mount, A.S. Chitin Facilitated Mineralization in the Eastern Oyster. Front. Mar. Sci. 2018, 5, 347. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H.; Luczak, M.; Ziganshin, R.; Mikšík, I.; Wysokowski, M.; Simon, P.; Baranowska-Bosiacka, I.; Kupnicka, P.; Ereskovsky, A.; Galli, R.; et al. Arrested in Glass: Actin within Sophisticated Architectures of Biosilica in Sponges. Adv. Sci. 2022, 9, 2105059. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Lim, C.T.; Li, A.; Hairul Nizam, B.R.; Tan, E.P.S.; Seki, Y.; McKittrick, J. The role of organic intertile layer in abalone nacre. Mater. Sci. Eng. C 2009, 29, 2398–2410. [Google Scholar] [CrossRef]
- Checa, A.G.; Cartwright, J.H.E.; Willinger, M.G. Mineral bridges in nacre. J. Struct. Biol. 2011, 176, 330–339. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [Green Version]
- Bewernitz, M.A.; Gebauer, D.; Long, J.; Cölfen, H.; Gower, L.B. A metastable liquid precursor phase of calcium carbonate and its interactions with polyaspartate. Faraday Discuss. 2012, 159, 291. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.E.; Gower, L.B. Challenges and Perspectives of the Polymer-Induced Liquid-Precursor Process: The Pathway from Liquid-Condensed Mineral Precursors to Mesocrystalline Products. New Perspect. Miner. Nucl. Growth 2017, 3, 43–75. [Google Scholar] [CrossRef]
- Gower, L.; Elias, J. Colloid assembly and transformation (CAT): The relationship of PILP to biomineralization. J. Struct. Biol. X 2022, 6, 100059. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskey, A.L.; Villarreal-Ramirez, E. Intrinsically disordered proteins and biomineralization. Matrix Biol. 2016, 52–54, 43–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kröger, R.; Estroff, L.A.; Evans, J.S. The Intrinsically Disordered C-RING Biomineralization Protein, AP7, Creates Protein Phases That Introduce Nanopatterning and Nanoporosities into Mineral Crystals. Biochemistry 2014, 53, 4317–4319. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.; Drechsler, M.; Schiller, S.; Scheffner, M.; Gebauer, D.; Cölfen, H. Stabilization of Mineral Precursors by Intrinsically Disordered Proteins. Adv. Funct. Mater. 2018, 28, 1802063. [Google Scholar] [CrossRef] [Green Version]
- Pendola, M.; Jain, G.; Evans, J.S. Skeletal development in the sea urchin relies upon protein families that contain intrinsic disorder, aggregation-prone, and conserved globular interactive domains. PLoS ONE 2019, 14, e0222068. [Google Scholar] [CrossRef]
- Wojtas, M.; Dobryszycki, P.; Ożyhar, A. Intrinsically Disordered Proteins in Biomineralization. Adv. Top. Biominer. 2012, 1, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Fisher, L.W.; Torchia, D.A.; Fohr, B.; Young, M.F.; Fedarko, N.S. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 2001, 280, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Nagasawa, H.; Luquet, G. The crustacean cuticle: Structure, composition and mineralization. Front. Biosci.-Elite 2012, 4, 711–720. [Google Scholar] [CrossRef]
- Aizenberg, J.; Addadi, L.; Weiner, S.; Lambert, G. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226. [Google Scholar] [CrossRef]
- Luquet, G. Biomineralizations: Insights and prospects from crustaceans. Zookeys 2012, 176, 103–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 1996, 381, 56. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Douglas, E.P.; Gower, L.B. Patterning Inorganic (CaCO3) Thin Films via a Polymer-Induced Liquid-Precursor Process. Langmuir 2007, 23, 4862–4870. [Google Scholar] [CrossRef]
- Cheng, X.; Gower, L.B. Molding mineral within microporous hydrogels by a polymer-induced liquid-precursor (PILP) process. Biotechnol. Prog. 2006, 22, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gower, L.A.; Tirrell, D.A. Calcium carbonate films and helices grown in solutions of poly(aspartate). J. Cryst. Growth 1998, 191, 153–160. [Google Scholar] [CrossRef]
- Amos, F.F.; Sharbaugh, D.M.; Talham, D.R.; Gower, L.B.; Fricke, M.; Volkmer, D. Formation of Single-Crystalline Aragonite Tablets/Films via an Amorphous Precursor. Langmuir 2007, 23, 1988–1994. [Google Scholar] [CrossRef]
- Olszta, M.J.; Douglas, E.P.; Gower, L.B. Scanning Electron Microscopic Analysis of the Mineralization of Type I Collagen via a Polymer-Induced Liquid-Precursor (PILP) Process. Calcif. Tissue Int. 2003, 72, 583–591. [Google Scholar] [CrossRef]
- Ping, H.; Xie, H.; Wan, Y.; Zhang, Z.; Zhang, J.; Xiang, M.; Xie, J.; Wang, H.; Wang, W.; Fu, Z. Confinement controlled mineralization of calcium carbonate within collagen fibrils. J. Mater. Chem. B 2016, 4, 880–886. [Google Scholar] [CrossRef]
- Niu, L.-N.; Jiao, K.; Qi, Y.-P.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.-H.; Breschi, L.; Pashley, D.H.; Tay, F.R.; et al. Infiltration of Silica Inside Fibrillar Collagen. Angew. Chemie Int. Ed. 2011, 50, 11688–11691. [Google Scholar] [CrossRef] [Green Version]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; De With, G.; Sommerdijk, N.A.J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Qi, H.; Zhao, Q.; Liu, Y.; Zhang, Y.; Zhang, J.; Qi, H.; Zhao, Q.; Zhang, Y.; et al. Dual Function of Magnesium in Bone Biomineralization. Adv. Healthc. Mater. 2019, 8, 1901030. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.H.M.; Hercz, G.; Kandel, R.; Grynpas, M.D. Association between fluoride, magnesium, aluminum and bone quality in renal osteodystrophy. Bone 2004, 34, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Hand, C. Another sea anemone from California and the types of certain Californian anemones. J. Washingt. Acad. Sci. 1957, 47, 411–414. [Google Scholar]
- Schlüter, L.; Lohbeck, K.T.; Gutowska, M.A.; Gröger, J.P.; Riebesell, U.; Reusch, T.B.H. Adaptation of a globally important coccolithophore to ocean warming and acidification. Nat. Clim. Chang. 2014, 4, 1024–1030. [Google Scholar] [CrossRef]
- Nishino, Y.; Oaki, Y.; Imai, H. Magnesium-mediated nanocrystalline mosaics of calcite. Cryst. Growth Des. 2009, 9, 223–226. [Google Scholar] [CrossRef]
- Macías-Sánchez, E.; Tarakina, N.V.; Ivanov, D.; Blouin, S.; Berzlanovich, A.M.; Fratzl, P. Spherulitic Crystal Growth Drives Mineral Deposition Patterns in Collagen-Based Materials. Adv. Funct. Mater. 2022, 2200504. [Google Scholar] [CrossRef]
- Olszta, M.J.; Gajjeraman, S.; Kaufman, M.; Gower, L.B. Nanofibrous Calcite Synthesized via a Solution-Precursor-Solid Mechanism. Chem. Mater. 2004, 16, 2355–2362. [Google Scholar] [CrossRef]
- Homeijer, S.J.; Olszta, M.J.; Barrett, R.A.; Gower, L.B. Growth of nanofibrous barium carbonate on calcium carbonate seeds. J. Cryst. Growth 2008, 310, 2938–2945. [Google Scholar] [CrossRef]
- Politi, Y.; Batchelor, D.R.; Zaslansky, P.; Chmelka, B.F.; Weaver, J.C.; Sagi, I.; Weiner, S.; Addadi, L. Role of Magnesium Ion in the Stabilization of Biogenic Amorphous Calcium Carbonate: A Structure−Function Investigation. Chem. Mater. 2010, 22, 161–166. [Google Scholar] [CrossRef]
- Cheng, X.; Varona, P.L.; Olszta, M.J.; Gower, L.B. Biomimetic synthesis of calcite films by a polymer-induced liquid-precursor (PILP) process: 1. Influence and incorporation of magnesium. J. Cryst. Growth 2007, 307, 395–404. [Google Scholar] [CrossRef]
- Loste, E.; Wilson, R.M.; Seshadri, R.; Meldrum, F.C. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J. Cryst. Growth 2003, 254, 206–218. [Google Scholar] [CrossRef]
- Niu, L.; Jee, S.E.; Jiao, K.; Tonggu, L.; Li, M.; Wang, L.; Yang, Y.; Bian, J.; Breschi, L.; Jang, S.S.; et al. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat. Mater. 2017, 16, 370–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bewernitz, M.A.; Lovett, A.C.; Gower, L.B. Liquid–Solid Core-Shell Microcapsules of Calcium Carbonate Coated Emulsions and Liposomes. Appl. Sci. 2020, 10, 8551. [Google Scholar] [CrossRef]
- Antao, S.M. Temperature dependence of the structural parameters in the transformation of aragonite to calcite, as determined from in situ synchrotron powder x-ray-diffraction data. Can. Mineral. 2010, 48, 1225–1236. [Google Scholar] [CrossRef] [Green Version]






| Component | Concentration (mM) | |||
|---|---|---|---|---|
| Instant Ocean Seawater Mix | 1/2x ASW | NaCl + 10 mM Ca | NaCl + 10 mM Ca + 50 mM Mg | |
| Chloride | 544 | 272 | 160 | 160 |
| Sodium | 469 | 235 | 190 | 190 |
| Sulfate | 28 | 14 | 20 | 20 |
| Magnesium | 54 | 27 | 0 | 50 |
| Potassium | 10 | 5 | 10 | 10 |
| Calcium | 10 | 5 | 10 | 10 |
| Carbonate/bicarbonate | 3 | 2 | 0 * | 0 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elias, J.; Angelini, T.; Martindale, M.Q.; Gower, L. Assessment of Optimal Conditions for Marine Invertebrate Cell-Mediated Mineralization of Organic Matrices. Biomimetics 2022, 7, 86. https://doi.org/10.3390/biomimetics7030086
Elias J, Angelini T, Martindale MQ, Gower L. Assessment of Optimal Conditions for Marine Invertebrate Cell-Mediated Mineralization of Organic Matrices. Biomimetics. 2022; 7(3):86. https://doi.org/10.3390/biomimetics7030086
Chicago/Turabian StyleElias, Jeremy, Thomas Angelini, Mark Q. Martindale, and Laurie Gower. 2022. "Assessment of Optimal Conditions for Marine Invertebrate Cell-Mediated Mineralization of Organic Matrices" Biomimetics 7, no. 3: 86. https://doi.org/10.3390/biomimetics7030086
APA StyleElias, J., Angelini, T., Martindale, M. Q., & Gower, L. (2022). Assessment of Optimal Conditions for Marine Invertebrate Cell-Mediated Mineralization of Organic Matrices. Biomimetics, 7(3), 86. https://doi.org/10.3390/biomimetics7030086