Diversity of Mycogenic Oxide and Chalcogenide Nanoparticles: A Review
Abstract
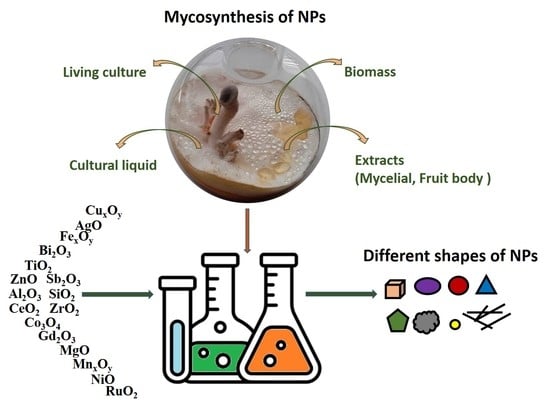
:1. Introduction
2. Myco-Synthesis of Oxide Nanoparticles
2.1. Myco-Synthesis of Copper Oxide Nanoparticles
2.2. Myco-Synthesis of Iron Oxide Nanoparticles
2.3. Titanium Oxide NPs
2.4. Myco-Synthesis of Zinc Oxide Nanoparticles
2.5. Myco-Synthesis of Nanoparticles of Other Elements
3. Myco-Synthesis of Chalcogenide Nanoparticles
3.1. Myco-Synthesis of Sulfide Nanoparticles
3.2. Myco-Synthesis of Selenide Nanoparticles
3.3. Myco-Synthesis of Telluride Nanoparticles
4. Prospects of Mycogenic Oxide and Chalcogenide Nanoparticles Practical Application
5. Conclusions
- screening fungal cultures to identify NP producers of new, previously unexplored compounds;
- further enhancing the knowledge of already known mycogenic oxide and chalcogenide NPs;
- optimization of production methods and scaling up of processes for the biosynthesis of NPs with the required properties on an industrial scale;
- studying the possibilities of practical application of NPs and their introduction into practice.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ovais, M.; Khalil, A.; Ayaz, M.; Ahmad, I.; Nethi, S.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Zane, D.; Dragone, R. Microbial Nanotechnology: Challenges and Prospects for Green Biocatalytic Synthesis of Nanoscale Materials for Sensoristic and Biomedical Applications. Nanomaterials 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A. Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review. Biomimetics 2023, 8, 1. [Google Scholar] [CrossRef]
- Castro-Longoria, E. Fungal Biosynthesis of Nanoparticles, a Cleaner Alternative. In Fungal Applications in Sustainable Environmental Biotechnology; Purchase, D., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 323–351. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Azeez, M.A.; Alao, M.B.; Oke, A.M.; Aina, D.A. Fungi as veritable tool in current advances in nanobiotechnology. Heliyon 2021, 7, e08480. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Li, M.; Chen, C.; Gadd, G.M. Nanoparticle and nanomineral production by fungi. Fungal Biol. Rev. 2022, 41, 31–44. [Google Scholar] [CrossRef]
- Zielonka, A.; Klimek-Ochab, M. Fungal synthesis of size-defined nanoparticles. Adv. Nat. Sci: Nanosci. Nanotechnol. 2017, 8, 043001. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Nikitina, V. Shape and Size Diversity of Gold, Silver, Selenium, and Silica Nanoparticles Prepared by Green Synthesis Using Fungi and Bacteria. Ind. Eng. Chem. Res. 2019, 58, 17207–17218. [Google Scholar] [CrossRef]
- Qu, M.; Yao, W.; Cui, X.; Xia, R.; Qin, L.; Liu, X. Biosynthesis of silver nanoparticles (AgNPs) employing Trichoderma strains to control empty-gut disease of oak silkworm (Antheraea pernyi). Mater. Today Commun. 2021, 28, 102619. [Google Scholar] [CrossRef]
- Mal, J.; Nancharaiah, Y.V.; Van Hullebusch, E.D.; Lens, P.N.L. Metal chalcogenide quantum dots: Biotechnological synthesis and applications. RSC Adv. 2016, 6, 41477–41495. [Google Scholar] [CrossRef]
- Chouke, P.B.; Shrirame, T.; Potbhare, A.K.; Mondal, A.; Chaudhary, A.R.; Mondal, S.; Thakare, S.R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Bioinspired metal/metal oxide nanoparticles: A road map to potential applications. Mater. Today Adv. 2022, 16, 100314. [Google Scholar] [CrossRef]
- Campos, E.A.; Stockler Pinto, D.V.B.; Oliveira, J.I.S.D.; Mattos, E.D.C.; Dutra, R.D.C.L. Synthesis, Characterization and Applications of Iron Oxide Nanoparticles—A Short Review. J. Aerosp. Technol. Manag. 2015, 7, 267–276. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Baloch, H.A.; Abdullah, E.C.; Mazari, S.A.; Griffin, G.J.; Srinivasan, M.P.; Tanksale, A. Iron Oxide Nanomaterials for the Removal of Heavy Metals and Dyes From Wastewater. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–472. ISBN 978-0-12-813926-4. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G. New frontiers in the biosynthesis of metal oxide nanoparticles and their environmental applications: An overview. SN Appl. Sci. 2019, 1, 928. [Google Scholar] [CrossRef]
- Marouzi, S.; Sabouri, Z.; Darroudi, M. Greener synthesis and medical applications of metal oxide nanoparticles. Ceram. Int. 2021, 47, 19632–19650. [Google Scholar] [CrossRef]
- Freitas, J.N.; Gonçalves, A.S.; Nogueira, A.F. A comprehensive review of the application of chalcogenide nanoparticles in polymer solar cells. Nanoscale 2014, 6, 6371–6397. [Google Scholar] [CrossRef]
- Feng, Y.; Marusak, K.E.; You, L.; Zauscher, S. Biosynthetic transition metal chalcogenide semiconductor nanoparticles: Progress in synthesis, property control and applications. Curr. Opin. Colloid Interface Sci. 2018, 38, 190–203. [Google Scholar] [CrossRef]
- Olawale, F.; Oladimeji, O.; Ariatti, M.; Singh, M. Emerging Roles of Green-Synthesized Chalcogen and Chalcogenide Nanoparticles in Cancer Theranostics. J. Nanotechnol. 2022, 2022, 6176610. [Google Scholar] [CrossRef]
- Bonilla, C.A.M.; Kouznetsov, V.V. “Green” Quantum Dots: Basics, Green Synthesis, and Nanotechnological Applications. In Green Nanotechnology—Overview and Further Prospects; Larramendy, M.L., Soloneski, S., Eds.; InTech: Vienna, Austria, 2016; ISBN 978-953-51-2409-2. [Google Scholar] [CrossRef]
- Farzin, M.A.; Abdoos, H. A critical review on quantum dots: From synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, G.; Rawat, M. A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications. J. Bioelectron. Nanotechnol. 2016, 1, 9. [Google Scholar]
- Verma, N.; Kumar, N. Synthesis and Biomedical Applications of Copper Oxide Nanoparticles: An Expanding Horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; EL-Aasser, M.M.; Ayoub, S.M.; EL- Shiekh, H.H.; Sakr, T.M. Exploitation of Aspergillus flavus synthesized copper oxide nanoparticles as a novel medical agent. J. Radioanal. Nucl. Chem. 2021, 328, 299–313. [Google Scholar] [CrossRef]
- Mousa, S.A.; El-Sayed, E.-S.R.; Mohamed, S.S.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Abdou, D.A.M. Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic Aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl. Microbiol. Biotechnol. 2021, 105, 741–753. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.R.; Mousa, S.A.; Abdou, D.A.M.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Mohamed, S.S. Exploiting the exceptional biosynthetic potency of the endophytic Aspergillus terreus in enhancing production of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles using bioprocess optimization and gamma irradiation. Saudi J. Biol. Sci. 2022, 29, 2463–2474. [Google Scholar] [CrossRef]
- Mani, V.M.; Kalaivani, S.; Sabarathinam, S.; Vasuki, M.; Soundari, A.J.P.G.; Ayyappa Das, M.P.; Elfasakhany, A.; Pugazhendhi, A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ. Res. 2021, 201, 111502. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for in vitro Antimicrobial Activity Against Some Plant Pathogens. J. Clust. Sci. 2020, 31, 79–90. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmugam, S.; Varukattu, N.B.; MubarakAli, D.; Kathiresan, K.; Wang, M.-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B 2019, 190, 103–109. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Cuevas, R.; Durán, N.; Diez, M.C.; Tortella, G.R.; Rubilar, O. Extracellular Biosynthesis of Copper and Copper Oxide Nanoparticles by Stereum hirsutum, a Native White-Rot Fungus from Chilean Forests. J. Nanomater. 2015, 16, 57. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei, E.; Naghibi, F. Green synthesis of copper oxide nanoparticles using Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii. Dig. J. Nanomater. Biostructures 2012, 7, 999–1005. [Google Scholar]
- Rana, S.; Sharma, S.; Kalia, A.; Kapoor, S. Functionalization with bio-molecules derived from oyster mushroom (Pleurotus florida) diminished the antibacterial potential of the mycogenic metal oxide nanoparticles (nps). Mushroom Res. 2021, 30, 77. [Google Scholar] [CrossRef]
- Bilesky-José, N.; Maruyama, C.; Germano-Costa, T.; Campos, E.; Carvalho, L.; Grillo, R.; Fraceto, L.F.; de Lima, R. Biogenic α-Fe2O3 Nanoparticles Enhance the Biological Activity of Trichoderma against the Plant Pathogen Sclerotinia sclerotiorum. ACS Sustain. Chem. Eng. 2021, 9, 1669–1683. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Mohamed, Y.M.; Azzam, A.M.; Amin, B.H.; Safwat, N.A. Mycosynthesis of iron nanoparticles by Alternaria alternata and its antibacterial activity. Afr. J. Biotechnol. 2015, 14, 1234–1241. [Google Scholar] [CrossRef]
- Abdeen, M.; Sabry, S.; Ghozlan, H.; El-Gendy, A.A.; Carpenter, E.E. Microbial-Physical Synthesis of Fe and Fe3O4 Magnetic Nanoparticles Using Aspergillus niger YESM1 and Supercritical Condition of Ethanol. J. Nanomater. 2016, 2016, 9174891. [Google Scholar] [CrossRef]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Chatterjee, S.; Bhattacharyya, S.; Das, P.; Das, S.; Chaudhuri, P. Green Synthesis of Iron Oxide Nanoparticles Mediated by Filamentous Fungi Isolated from Sundarban Mangrove Ecosystem, India. BioNanoScience 2019, 9, 637–651. [Google Scholar] [CrossRef]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Gaine, T.; Chatterjee, S.; Bhattacharyya, S.; Das, S.; Das, P.; Chaudhuri, P. Mycosynthesis of iron oxide nanoparticles using manglicolous fungi isolated from Indian sundarbans and its application for the treatment of chromium containing solution: Synthesis, adsorption isotherm, kinetics and thermodynamics study. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100276. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mahanty, S.; Das, P.; Chaudhuri, P.; Das, S. Biofabrication of iron oxide nanoparticles using manglicolous fungus Aspergillus niger BSC-1 and removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2020, 385, 123790. [Google Scholar] [CrossRef]
- El-Sharkawy, R.M.; Swelim, M.A.; Hamdy, G.B. Aspergillus tamarii mediated green synthesis of magnetic chitosan beads for sustainable remediation of wastewater contaminants. Sci. Rep. 2022, 12, 9742. [Google Scholar] [CrossRef]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular Biosynthesis of Magnetite using Fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sasani, M.; Fataei, E.; Safari, R.; Nasehi, F.; Mosayyebi, M. Antimicrobial Potentials of Iron Oxide and Silver Nanoparticles Green-Synthesized in Fusarium solani. J. Chem. Health Risks 2023, 13, 95–104. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Przysiecka, Ł.; Peplińska, B.; Jarek, M.; Coy, E.; Jurga, S. Gel with silver and ultrasmall iron oxide nanoparticles produced with Amanita muscaria extract: Physicochemical characterization, microstructure analysis and anticancer properties. Sci. Rep. 2018, 8, 13260. [Google Scholar] [CrossRef]
- Bhargava, A.; Jain, N.; Barathi, L.M.; Akhtar, M.S.; Yun, Y.S.; Panwar, J. Synthesis, characterization and mechanistic insights of mycogenic iron oxide nanoparticles. J. Nanopart. Res. 2013, 15, 2031. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Liu, Z.; Gao, Y.; Wu, B.; Xu, H. Fe3O4 nanoparticle-coated mushroom source biomaterial for Cr(VI) polluted liquid treatment and mechanism research. R. Soc. Open Sci. 2018, 5, 171776. [Google Scholar] [CrossRef] [PubMed]
- Ince, O.K.; Aydogdu, B.; Alp, H.; Ince, M. Experimental design approach for ultra-fast nickel removal by novel bio-nanocomposite material. Adv. Nano Res. 2021, 10, 77–90. [Google Scholar] [CrossRef]
- Ince, M.; Ince, O.K.; Aydogdu, B.; Alp, H. Green-synthesis of superparamagnetic Fe3O4/alginate bio-nanocomposites for heavy metal contamination removal from industrial wastewater. Res. Sq. 2022, 3, 1–18. [Google Scholar] [CrossRef]
- Shiva Samhitha, S.; Raghavendra, G.; Quezada, C.; Hima Bindu, P. Green synthesized TiO2 nanoparticles for anticancer applications: Mini review. Mater. Today Proc. 2022, 54, 765–770. [Google Scholar] [CrossRef]
- Sagadevan, S.; Imteyaz, S.; Murugan, B.; Lett, J.A.; Sridewi, N.; Weldegebrieal, G.K.; Fatimah, I.; Oh, W.-C. A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process. Synth. 2022, 11, 44–63. [Google Scholar] [CrossRef]
- Jaffer Al-Timimi, I.A.; Sermon, P.A.; Burghal, A.A.; Salih, A.A.; Alrubaya, I.M.N. Nanoengineering the antibacterial activity of biosynthesized nanoparticles of TiO2, Ag, and Au and their nanohybrids with Portobello mushroom spore (PMS) (TiOx/PMS, Ag/PMS and Au/PMS) and making them optically self-indicating. In Biosensing and Nanomedicine IX; Mohseni, H., Agahi, M.H., Razeghi, M., Eds.; Proc. of SPIE: San Diego, CA, USA, 2016; Volume 9930, p. 99300B. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, B.; Xavier, T.; Muthu, S. Fungal Generated Titanium Dioxide Nanoparticles for UV Protective and Bacterial Resistant Fabrication. Int. J. Eng. Sci. Technol. 2014, 6, 621–625. [Google Scholar]
- Tarafdar, A.; Raliya, R.; Wang, W.-N.; Biswas, P.; Tarafdar, J.C. Green Synthesis of TiO2 Nanoparticle Using Aspergillus tubingensis. Adv. Sci. Engng. Med. 2013, 5, 943–949. [Google Scholar] [CrossRef]
- Rehman, S.; Farooq, R.; Jermy, R.; Mousa Asiri, S.; Ravinayagam, V.; Al Jindan, R.; Alsalem, Z.; Shah, M.A.; Reshi, Z.; Sabit, H.; et al. A Wild Fomes fomentarius for Biomediation of One Pot Synthesis of Titanium Oxide and Silver Nanoparticles for Antibacterial and Anticancer Application. Biomolecules 2020, 10, 622. [Google Scholar] [CrossRef]
- Rehman, S.; Jermy, R.; Mousa Asiri, S.; Shah, M.A.; Farooq, R.; Ravinayagam, V.; Azam Ansari, M.; Alsalem, Z.; Al Jindan, R.; Reshi, Z.; et al. Using Fomitopsis pinicola for bioinspired synthesis of titanium dioxide and silver nanoparticles, targeting biomedical applications. RSC Adv. 2020, 10, 32137–32147. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmad, A. Phase, size and shape transformation by fungal biotransformation of bulk TiO2. Chem. Eng. J. 2013, 230, 367–371. [Google Scholar] [CrossRef]
- Manimaran, K.; Loganathan, S.; Prakash, D.G.; Natarajan, D. Antibacterial and anticancer potential of mycosynthesized titanium dioxide (TiO2) nanoparticles using Hypsizygus ulmarius. Biomass Conv. Bioref. 2022, 1–9. [Google Scholar] [CrossRef]
- Manimaran, K.; Murugesan, S.; Ragavendran, C.; Balasubramani, G.; Natarajan, D.; Ganesan, A.; Seedevi, P. Biosynthesis of TiO2 Nanoparticles Using Edible Mushroom (Pleurotus djamor) Extract: Mosquito Larvicidal, Histopathological, Antibacterial and Anticancer Effect. J. Clust. Sci. 2021, 32, 1229–1240. [Google Scholar] [CrossRef]
- Manimaran, K.; Natarajan, D.; Balasubramani, G.; Murugesan, S. Pleurotus sajor caju Mediated TiO2 Nanoparticles: A Novel Source for Control of Mosquito Larvae, Human Pathogenic Bacteria and Bone Cancer Cells. J. Clust. Sci. 2022, 33, 1489–1499. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Kulkarni, A.R. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf. B Biointerfaces 2009, 71, 226–229. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cui, J.; Yue, Y.; Zhang, X.; Xia, X.; Liu, H.; Lui, S. High-performance TiO2 from Baker’s yeast. J. Colloid Interface Sci. 2011, 354, 109–115. [Google Scholar] [CrossRef]
- Peiris, M.; Gunasekara, T.; Jayaweera, P.; Fernando, S. TiO2 Nanoparticles from Baker’s yeast: A Potent Antimicrobial. J. Microbiol. Biotechnol. 2018, 28, 1664–1670. [Google Scholar] [CrossRef]
- Arya, S.; Sonawane, H.; Math, S.; Tambade, P.; Chaskar, M.; Shinde, D. Biogenic titanium nanoparticles (TiO2NPs) from Tricoderma citrinoviride extract: Synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int. Nano Lett. 2021, 11, 35–42. [Google Scholar] [CrossRef]
- Chinnaperumal, K.; Govindasamy, B.; Paramasivam, D.; Dilipkumar, A.; Dhayalan, A.; Vadivel, A.; Sengodan, K.; Pachiappan, P. Bio-pesticidal effects of Trichoderma viride formulated titanium dioxide nanoparticle and their physiological and biochemical changes on Helicoverpa armigera (Hub.). Pestic. Biochem. Physiol. 2018, 149, 26–36. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Ameen, F.; Dawoud, T.; AlNadhari, S. Ecofriendly and low-cost synthesis of ZnO nanoparticles from Acremonium potronii for the photocatalytic degradation of azo dyes. Environ. Res. 2021, 202, 111700. [Google Scholar] [CrossRef]
- Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Abilaji, S.; Prakash, C.; Rajasekar, A.; Nanthini, A.U.R.; Valli, G. Myco-Synthesis of Zinc Oxide Nanoparticles as Potent Anti-corrosion of Copper in Cooling Towers. J. Clust. Sci. 2019, 30, 1583–1590. [Google Scholar] [CrossRef]
- Jain, N.; Bhargava, A.; Tarafdar, J.C.; Singh, S.K.; Panwar, J. A biomimetic approach towards synthesis of zinc oxide nanoparticles. Appl. Microbiol. Biotechnol. 2013, 97, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Cherian, E.; Baskar, G. Biosynthesis of zinc oxide nanoparticles using Aspergillus fumigatus JCF and its antibacterial activity. Int. J. Mod. Sci. Technol. 2016, 1, 52–57. [Google Scholar]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Shamim, A.; Mahmood, T.; Abid, M.B. Biogenic Synthesis of Zinc Oxide (ZnO) Nanoparticles Using a Fungus (Aspargillus niger) and Their Characterization. Int. J. Chem. 2019, 11, 119. [Google Scholar] [CrossRef]
- Baskar, G.; Chandhuru, J.; Sheraz Fahad, K.; Praveen, A.S.; Chamundeeswari, M.; Muthukumar, T. Anticancer activity of fungal L-asparaginase conjugated with zinc oxide nanoparticles. J. Mater. Sci. Mater. Med. 2015, 26, 43. [Google Scholar] [CrossRef]
- Fouda, A.; EL-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Kadam, V.V.; Ettiyappan, J.P.; Mohan Balakrishnan, R. Mechanistic insight into the endophytic fungus mediated synthesis of protein capped ZnO nanoparticles. Mater. Sci. Eng. B 2019, 243, 214–221. [Google Scholar] [CrossRef]
- Dias, C.; Ayyanar, M.; Amalraj, S.; Khanal, P.; Subramaniyan, V.; Das, S.; Gandhale, P.; Biswa, V.; Ali, R.; Gurav, N.; et al. Biogenic synthesis of zinc oxide nanoparticles using mushroom fungus Cordyceps militaris: Characterization and mechanistic insights of therapeutic investigation. J. Drug Deliv. Sci. Technol. 2022, 73, 103444. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Ullah, K.; Almutairi, S.M.; AlMunqedhi, B.M.; Ragab abdelGawwad, M. Mycosynthesis, Characterization of Zinc Oxide Nanoparticles, and Its Assessment in Various Biological Activities. Crystals 2023, 13, 171. [Google Scholar] [CrossRef]
- Chauhan, N.; Thakur, N.; Kumari, A.; Khatana, C.; Sharma, R. Mushroom and silk sericin extract mediated ZnO nanoparticles for removal of organic pollutants and microorganisms. S. Afr. J. Bot. 2023, 153, 370–381. [Google Scholar] [CrossRef]
- Ganesan, V.; Hariram, M.; Vivekanandhan, S.; Muthuramkumar, S. Periconium sp. (endophytic fungi) extract mediated sol-gel synthesis of ZnO nanoparticles for antimicrobial and antioxidant applications. Mater. Sci. Semicond. Process. 2020, 105, 104739. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Rafeeq, C.M.; Paul, E.; Vidya Saagar, E.; Manzur Ali, P.P. Mycosynthesis of zinc oxide nanoparticles using Pleurotus floridanus and optimization of process parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Pooe, O.J.; Khoza, S.; Mongalo, I.N.; Khan, R.; Simelane, M.B.C. Characterization and Biological Evaluation of Zinc Oxide Nanoparticles Synthesized from Pleurotus ostreatus Mushroom. Appl. Sci. 2022, 12, 8563. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Jeevithan, E.; Hu, X.; Chelliah, R.; Oh, D.-H.; Wang, M.-H. Enhanced anti-lung carcinoma and anti-biofilm activity of fungal molecules mediated biogenic zinc oxide nanoparticles conjugated with β-D-glucan from barley. J. Photochem. Photobiol. B 2020, 203, 111728. [Google Scholar] [CrossRef]
- Kaur, T.; Bala, M.; Kumar, G.; Vyas, A. Biosynthesis of zinc oxide nanoparticles via endophyte Trichoderma viride and evaluation of their antimicrobial and antioxidant properties. Arch. Microbiol. 2022, 204, 620. [Google Scholar] [CrossRef]
- Chauhan, R.; Reddy, A.; Abraham, J. Biosynthesis of silver and zinc oxide nanoparticles using Pichia fermentans JA2 and their antimicrobial property. Appl. Nanosci. 2015, 5, 63–71. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.- mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT Food Sci. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Vellingiri, M.M.; Ashwin, J.K.M.; Soundari, A.J.P.G.; Sathiskumar, S.; Priyadharshini, U.; Paramasivam, D.; Liu, W.-C.; Balasubramanian, B. Mycofabrication of AgONPs derived from Aspergillus terreus FC36AY1 and its potent antimicrobial, antioxidant, and anti-angiogenesis activities. Mol. Biol. Rep. 2021, 48, 7933–7946. [Google Scholar] [CrossRef]
- Uddin, I.; Adyanthaya, S.; Syed, A.; Selvaraj, K.; Ahmad, A.; Poddar, P. Structure and Microbial Synthesis of Sub-10 nm Bi2O3 Nanocrystals. J. Nanosci. Nanotechnol. 2008, 8, 3909–3913. [Google Scholar] [CrossRef]
- Gopinath, K.; Karthika, V.; Sundaravadivelan, C.; Gowri, S.; Arumugam, A. Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J. Nanostruct. Chem. 2015, 5, 295–303. [Google Scholar] [CrossRef]
- Komal, R.; Uzair, B.; Sajjad, S.; Butt, S.; Kanwal, A.; Ahmed, I.; Riaz, N.; Leghari, S.A.K.; Abbas, S. Skirmishing MDR strain of Candida albicans by effective antifungal CeO2 nanostructures using Aspergillus terreus and Talaromyces purpurogenus. Mater. Res. Express 2020, 7, 055004. [Google Scholar] [CrossRef]
- Venkatesh, K.S.; Gopinath, K.; Palani, N.S.; Arumugam, A.; Jose, S.P.; Bahadur, S.A.; Ilangovan, R. Plant pathogenic fungus Fusarium solani mediated biosynthesis of nanoceria: Antibacterial and antibiofilm activity. RSC Adv. 2016, 6, 42720–42729. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmad, A. Fungus mediated synthesis of biomedically important cerium oxide nanoparticles. Mater. Res. Bull. 2013, 48, 4134–4138. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; El-Salamony, R.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404—Optimization, characterization and antimicrobial activity. J. Appl. Microbiol. 2020, 128, 438–457. [Google Scholar] [CrossRef]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Gambhir, S.; Ahmad, A. Extracellular biosynthesis of gadolinium oxide (Gd2O3) nanoparticles, their biodistribution and bioconjugation with the chemically modified anticancer drug taxol. Beilstein J. Nanotechnol. 2014, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech 2017, 7, 263. [Google Scholar] [CrossRef]
- Ibrahem, E.; Thalij, K.; Badawy, A. Antibacterial Potential of Magnesium Oxide Nanoparticles Synthesized by Aspergillus niger. Biotechnol. J. Int. 2017, 18, 1–7. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Choudhary, K.; Mal, P.; Raturi, A.; Gautam, R.; Singh, S.K. Synthesis of MgO Nanoparticles Using Aspergillus tubingensis TFR-3. J. Bionanosci. 2014, 8, 34–38. [Google Scholar] [CrossRef]
- Alrabadi, N.I.; Thalij, K.M.; Hussein, E.I.; Al-Trad, B.M. Antibacterial Activity of Ag and MgO Nanoparticles Synthesized by Trichoderma viride. J. Appl. Environ. Biol. Sci. 2017, 7, 94–101. [Google Scholar]
- Wang, M.; Xu, Z.; Dong, B.; Zeng, Y.; Chen, S.; Zhang, Y.; Huang, Y.; Pei, X. An efficient manganese-oxidizing fungus Cladosporium halotolerans strain XM01: Mn(II) oxidization and Cd adsorption behavior. Chemosphere 2022, 287, 132026. [Google Scholar] [CrossRef]
- Uddin, I.; Poddar, P.; Ahmad, A. Extracellular Biosynthesis of Water Dispersible, Protein Capped Mn5O8 Nanoparticles Using the Fungus Fusarium oxysporum and Study of Their Magnetic Behavior. J. Nanoeng. Nanomanuf. 2013, 3, 91–97. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Nascimento, C.A.O.; Corrêa, B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci. Rep. 2014, 4, 6404. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Ando, R.A.; Oller Nascimento, C.A.; Corrêa, B. Extra and Intracellular Synthesis of Nickel Oxide Nanoparticles Mediated by Dead Fungal Biomass. PLoS ONE 2015, 10, e0129799. [Google Scholar] [CrossRef]
- Parveen, S.; Najrul Islam, S.; Ahmad, A. Mycological synthesis of Ruthenium oxide quantum dots and their application in the colorimetric detection of H2O2. Adv. Powder Technol. 2022, 33, 103861. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K. A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 2009, 43, 303–306. [Google Scholar] [CrossRef]
- Liang, X.; Perez, M.A.M.-J.; Nwoko, K.C.; Egbers, P.; Feldmann, J.; Csetenyi, L.; Gadd, G.M. Fungal formation of selenium and tellurium nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 7241–7259. [Google Scholar] [CrossRef]
- Bansal, V.; Ahmad, A.; Sastry, M. Fungus-Mediated Biotransformation of Amorphous Silica in Rice Husk to Nanocrystalline Silica. J. Am. Chem. Soc. 2006, 128, 14059–14066. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; Jafari, A.; Mousavi, S.M.; Darezereshki, E. Biosynthesis of silica nanoparticle using Saccharomyces cervisiae and its application on enhanced oil recovery. J. Pet. Sci. Eng. 2020, 190, 107002. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004, 14, 3303–3305. [Google Scholar] [CrossRef]
- Kavitha, N.S.; Venkatesh, K.S.; Palani, N.S.; Ilangovan, R. Synthesis and Characterization of Zirconium Oxide Nanoparticles Using Fusarium Solani Extract; AIP Conference Proceedings: Jodhpur, India, 2020; Volume 2265, p. 030057. [Google Scholar] [CrossRef]
- Golnaraghi Ghomi, A.R.; Mohammadi-Khanaposhti, M.; Vahidi, H.; Kobarfard, F.; Ameri Shah Reza, M.; Barabadi, H. Fungus-mediated Extracellular Biosynthesis and Characterization of Zirconium Nanoparticles Using Standard Penicillium Species and Their Preliminary Bactericidal Potential: A Novel Biological Approach to Nanoparticle Synthesis. Iran. J. Pharm. Res. 2019, 18, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Se, S.; Ahluwalia, G.K.T. (Eds.) Applications of Chalcogenides; Springer International Publishing: Cham, Switzerland, 2017; 461p. [Google Scholar] [CrossRef]
- Syed, A.; Al Saedi, M.H.; Bahkali, A.H.; Elgorban, A.M.; Kharat, M.; Pai, K.; Ghodake, G.; Ahmad, A. Biological synthesis of α-Ag2S composite nanoparticles using the fungus Humicola sp. and its biomedical applications. J. Drug Deliv. Sci. Technol. 2021, 66, 102770. [Google Scholar] [CrossRef]
- Syed, A.; Al Saedi, M.H.; Bahkali, A.H.; Elgorgan, A.M.; Kharat, M.; Pai, K.; Pichtel, J.; Ahmad, A. αAu 2 S nanoparticles: Fungal-mediated synthesis, structural characterization and bioassay. Green Chem. Lett. Rev. 2022, 15, 61–70. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Elbaz, A.F.; El Badawy-, S.; Moussa, S.H. Anticancer and Antibacterial Activity of Cadmium Sulfide Nanoparticles by Aspergillus niger. Adv. Polym. Technol. 2020, 2020, 4909054. [Google Scholar] [CrossRef]
- Krumov, N.; Oder, S.; Perner-Nochta, I.; Angelov, A.; Posten, C. Accumulation of CdS nanoparticles by yeasts in a fed-batch bioprocess. J. Biotechnol. 2007, 132, 481–486. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Mandal, D.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Enzyme Mediated Extracellular Synthesis of CdS Nanoparticles by the Fungus, Fusarium oxysporum. J. Am. Chem. Soc. 2002, 124, 12108–12109. [Google Scholar] [CrossRef]
- Sandoval-Cárdenas, I.; Gómez-Ramírez, M.; Rojas-Avelizapa, N.G. Use of a sulfur waste for biosynthesis of cadmium sulfide quantum dots with Fusarium oxysporum f. sp. lycopersici. Mater. Sci. Semicond. Process. 2017, 63, 33–39. [Google Scholar] [CrossRef]
- Reyes, L.; Gomez, I.; Garza, M.T. Biosynthesis of Cadmium Sulfide Nanoparticles by the Fungi Fusarium sp. Int. J. Nanotechnol. Biomed. 2009, 1, 90–95. [Google Scholar] [CrossRef]
- Chen, G.; Yi, B.; Zeng, G.; Niu, Q.; Yan, M.; Chen, A.; Du, J.; Huang, J.; Zhang, Q. Facile green extracellular biosynthesis of CdS quantum dots by white rot fungus Phanerochaete chrysosporium. Colloids Surf. B. Biointerfaces 2014, 117, 199–205. [Google Scholar] [CrossRef]
- Borovaya, M.; Pirko, Y.; Krupodorova, T.; Naumenko, A.; Blume, Y.; Yemets, A. Biosynthesis of cadmium sulphide quantum dots by using Pleurotus ostreatus (Jacq.) P. Kumm. Biotechnol. Biotechnol. Equip. 2015, 29, 1156–1163. [Google Scholar] [CrossRef]
- Mareeswari, P.; Brijitta, J.; Harikrishna Etti, S.; Meganathan, C.; Kaliaraj, G.S. Rhizopus stolonifer mediated biosynthesis of biocompatible cadmium chalcogenide quantum dots. Enzyme. Microb. Technol. 2016, 95, 225–229. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. Biosynthesis of CdS nanoparticles: An improved green and rapid procedure. J. Colloid Interface Sci. 2010, 342, 68–72. [Google Scholar] [CrossRef]
- Wu, R.; Wang, C.; Shen, J.; Zhao, F. A role for biosynthetic CdS quantum dots in extracellular electron transfer of Saccharomyces cerevisiae. Process Biochem. 2015, 50, 2061–2065. [Google Scholar] [CrossRef]
- Williams, P.; Keshavarz-Moore, E.; Dunnill, P. Efficient production of microbially synthesized cadmium sulfide quantum semiconductor crystallites. Enzym. Microb. Technol. 1996, 19, 208–213. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef]
- Tudu, S.C.; Zubko, M.; Kusz, J.; Bhattacharjee, A. CdS nanoparticles (<5 nm): Green synthesized using Termitomyces heimii mushroom–structural, optical and morphological studies. Appl. Phys. A 2021, 127, 85. [Google Scholar] [CrossRef]
- Qin, Z.; Yue, Q.; Liang, Y.; Zhang, J.; Zhou, L.; Hidalgo, O.B.; Liu, X. Extracellular biosynthesis of biocompatible cadmium sulfide quantum dots using Trametes versicolor. J. Biotechnol. 2018, 284, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Bhadwal, A.S.; Tripathi, R.M.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv. 2014, 4, 9484–9490. [Google Scholar] [CrossRef]
- El-Baz, A.F.; Sorour, N.M.; Shetaia, Y.M. Trichosporon jirovecii—Mediated synthesis of cadmium sulfide nanoparticles: Biosynthesis of cadmium sulphide nanoparticles. J. Basic Microbiol. 2016, 56, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.R.; Schaffie, M.; Pazouki, M.; Darezereshki, E.; Ranjbar, M. Biologically synthesized copper sulfide nanoparticles: Production and characterization. Mater. Sci. Semicond. Process. 2012, 15, 222–225. [Google Scholar] [CrossRef]
- Schaffie, M.; Hosseini, M.R. Biological process for synthesis of semiconductor copper sulfide nanoparticle from mine wastewaters. J. Environ. Chem. Eng. 2014, 2, 386–391. [Google Scholar] [CrossRef]
- Priyanka, U.; KM, A.G.; Elisha, M.G.; Nitish, N. Biologically synthesized PbS nanoparticles for the detection of arsenic in water. Int. Biodeterior. Biodegrad. 2017, 119, 78–86. [Google Scholar] [CrossRef]
- Seshadri, S.; Saranya, K.; Kowshik, M. Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum. Biotechnol. Progress. 2011, 27, 1464–1469. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. PbS nanoparticles: Biosynthesis and characterisation. Int. J. Nanoparticles 2012, 5, 369–379. [Google Scholar] [CrossRef]
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial Synthesis of Semiconductor PbS Nanocrystallites. Adv. Mater. 2002, 14, 815–818. [Google Scholar] [CrossRef]
- Senapati, U.S.; Jha, D.K.; Sarkar, D. Structural, optical, thermal and electrical properties of fungus guided biosynthesized zinc sulphide nanoparticles. Res. J. Chem. Sci. 2015, 2231, 606X. [Google Scholar]
- Uddandarao, P. ZnS semiconductor quantum dots production by an endophytic fungus Aspergillus flavus. Mater. Sci. Eng. B 2016, 207, 26–32. [Google Scholar] [CrossRef]
- Uddandarao, P.; Balakrishnan, R.M. Thermal and optical characterization of biologically synthesized ZnS nanoparticles synthesized from an endophytic fungus Aspergillus flavus: A colorimetric probe in metal detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 175, 200–207. [Google Scholar] [CrossRef]
- Uddandarao, P.; Balakrishnan, R.M.; Ashok, A.; Swarup, S.; Sinha, P. Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection. Biomimetics 2019, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; Rajan, R.; Tom, T.C.; Kumar, V.S.; Kurup, G.G.; Shanmuganathan, R.; Pugazhendhi, A. Biogenic design of ZnS quantum dots—Insights into their in vitro cytotoxicity, photocatalysis and biosensing properties. Ceram. Int. 2019, 45, 24193–24201. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Darezereshki, E.; Bakhtiari, F.; Fazaelipoor, M.H.; Hosseini, M.R. Characterization of zinc sulfide (ZnS) nanoparticles Biosynthesized by Fusarium oxysporum. Mater. Sci. Semicond. Process. 2013, 16, 374–378. [Google Scholar] [CrossRef]
- Jacob, J.M.; Rajan, R.; Aji, M.; Kurup, G.G.; Pugazhendhi, A. Bio-inspired ZnS quantum dots as efficient photo catalysts for the degradation of methylene blue in aqueous phase. Ceram. Int. 2019, 45, 4857–4862. [Google Scholar] [CrossRef]
- Senapati, U.S.; Sarkar, D. Characterization of biosynthesized zinc sulphide nanoparticles using edible mushroom Pleurotus ostreatus. Indian J. Phys. 2014, 88, 557–562. [Google Scholar] [CrossRef]
- Sandana Mala, J.G.; Rose, C. Facile production of ZnS quantum dot nanoparticles by Saccharomyces cerevisiae MTCC 2918. J. Biotechnol. 2014, 170, 73–78. [Google Scholar] [CrossRef]
- Dameron, C.T.; Smith, B.R.; Winge, D.R. Glutathione-coated cadmium-sulfide crystallites in Candida glabrata. J. Biol. Chem. 1989, 264, 17355–17360. [Google Scholar] [CrossRef]
- Garmanchuk, L.V.; Borovaya, M.N.; Nehelia, A.O.; Inomistova, M.; Khranovska, N.M.; Tolstanova, G.M.; Blume, Y.B.; Yemets, A.I. CdS Quantum Dots Obtained by “Green” Synthesis: Comparative Analysis of Toxicity and Effects on the Proliferative and Adhesive Activity of Human Cells. Cytol. Genet. 2019, 53, 132–142. [Google Scholar] [CrossRef]
- Balakrishnan, R.M.; Kadam, V.V. Biological synthesis of metal selenide nanoparticles and their applications. In Environmental Technologies to Treat Selenium Pollution; Lens, P.N.L., Pakshirajan, K., Eds.; IWA Publishing: London, UK, 2021; pp. 323–351. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, D.; Zhong, L.; Gong, A.; Wu, S.; Xie, Z. Biosynthesis of biocompatibility Ag2Se quantum dots in Saccharomyces cerevisiae and its application. Biochem. Biophys. Res. Commun. 2021, 544, 60–64. [Google Scholar] [CrossRef]
- Islam, S.N.; Raza, A.; Naqvi, S.M.A.; Parveen, S.; Ahmad, A. Unveiling the antisporulant activity of mycosynthesized gold-selenide nanoparticles against black fungus Aspergillus niger. Surf. Interfac. 2022, 29, 101769. [Google Scholar] [CrossRef]
- Tian, L.-J.; Zhou, N.-Q.; Liu, X.-W.; Liu, J.-H.; Zhang, X.; Huang, H.; Zhu, T.-T.; Li, L.-L.; Huang, Q.; Li, W.-W.; et al. A Sustainable Biogenic Route to Synthesize Quantum Dots with Tunable Fluorescence Properties for Live Cell Imaging. Biochem. Eng. J. 2017, 124, 130–137. [Google Scholar] [CrossRef]
- Kumar, S.A.; Ansary, A.A.; Ahmad, A.; Khan, M.I. Extracellular Biosynthesis of CdSe Quantum Dots by the Fungus, Fusarium oxysporum. J. Biomed. Nanotechnol. 2007, 3, 190–194. [Google Scholar] [CrossRef]
- Suresh, A.K. Extracellular bio-production and characterization of small monodispersed CdSe quantum dot nanocrystallites. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Chen, M.-M.; Chang, F.-Y.; Cheng, Y.-Y.; Tian, L.-J.; Li, F.; Deng, G.-Z.; Wu, C. The biosynthesis of cadmium selenide quantum dots by Rhodotorula mucilaginosa PA-1 for photocatalysis. Biochem. Eng. J. 2020, 156, 107497. [Google Scholar] [CrossRef]
- Wu, S.-M.; Su, Y.; Liang, R.-R.; Ai, X.-X.; Qian, J.; Wang, C.; Chen, Q.; Yan, Z.-Y. Crucial factors in biosynthesis of fluorescent CdSe quantum dots in Saccharomyces cerevisiae. RSC Adv. 2015, 5, 79184–79191. [Google Scholar] [CrossRef]
- Brooks, J.; Lefebvre, D.D. Optimization of conditions for cadmium selenide quantum dot biosynthesis in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2735–2745. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, R.; Wang, C.; Hu, B.; Pang, D.; Xie, Z. Living cell synthesis of CdSe quantum dots: Manipulation based on the transformation mechanism of intracellular Se-precursors. Nano Res. 2018, 11, 2498–2511. [Google Scholar] [CrossRef]
- Sinharoy, A.; Lens, P.N.L. Indium removal by Aspergillus niger fungal pellets in the presence of selenite and tellurite. J. Water Process Eng. 2023, 51, 103421. [Google Scholar] [CrossRef]
- Ansary, A.A.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Varma, R.S.; Khan, M.S. Neodymium Selenide Nanoparticles: Greener Synthesis and Structural Characterization. Biomimetics 2022, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Mary Jacob, J.; Balakrishnan, R.M.; Kumar, U.B. Biosynthesis of lead selenide quantum rods in marine Aspergillus terreus. Mater. Lett. 2014, 124, 279–281. [Google Scholar] [CrossRef]
- Diko, C.S.; Qu, Y.; Henglin, Z.; Li, Z.; Ahmed Nahyoon, N.; Fan, S. Biosynthesis and characterization of lead selenide semiconductor nanoparticles (PbSe NPs) and its antioxidant and photocatalytic activity. Arab. J. Chem. 2020, 13, 8411–8423. [Google Scholar] [CrossRef]
- Luo, Q.-Y.; Lin, Y.; Li, Y.; Xiong, L.-H.; Cui, R.; Xie, Z.-X.; Pang, D.-W. Nanomechanical Analysis of Yeast Cells in CdSe Quantum Dot Biosynthesis. Small 2014, 10, 699–704. [Google Scholar] [CrossRef]
- Jamwal, D.; Mehta, S.K. Metal Telluride Nanomaterials: Facile Synthesis, Properties and Applications for Third Generation Devices. ChemistrySelect 2019, 4, 1943–1963. [Google Scholar] [CrossRef]
- Han, M.; Zhou, Z.; Li, Y.; Chen, Q.; Chen, M. Highly Conductive Tellurium and Telluride in Energy Storage. ChemElectroChem 2021, 8, 4412–4426. [Google Scholar] [CrossRef]
- Akbari, M.; Rahimi-Nasrabadi, M.; Eghbali-Arani, M.; Banafshe, H.R.; Ahmadi, F.; Ganjali, M.R. CdTe quantum dots prepared using herbal species and microorganisms and their anti-cancer, drug delivery and antibacterial applications; a review. Ceram. Int. 2020, 46, 9979–9989. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of CdTe quantum dots by the fungus Fusarium oxysporum and their anti-bacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Bao, H.; Hao, N.; Yang, Y.; Zhao, D. Biosynthesis of biocompatible cadmium telluride quantum dots using yeast cells. Nano Res. 2010, 3, 481–489. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P.; Gairola, S.P. Metal oxide nanoparticles and their nanocomposite-based materials as photocatalysts in the degradation of dyes. Biointerface Res. Appl. Chem. 2022, 12, 6557–6579. [Google Scholar]


| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| CuO | Aspergillus flavus | Culture liquid | CuSO4 | Spherical (average size of 32.4 nm) | [27] |
| CuO | Aspergillus terreus | Culture liquid | CuSO4 | – | [28,29] |
| CuO | Aspergillus terreus | Culture liquid | CuSO4 | Below 100 nm | [30] |
| CuO | Penicillium chrysogenum | Culture liquid | CuSO4 | Spherical (average size of 9.7 nm) | [31] |
| CuO | Trichoderma asperellum | Mycelial extract | Cu(NO3)2 | Spherical (10–190 nm) | [32] |
| CuO | Trichoderma harzianum | Mycelial extract | CuSO4 | Nano-fibers (38–77 nm in width, 135–320 nm in length) | [33] |
| Cu2O, CuO | Stereum hirsutum | Mycelial extract | CuCl2 | Spherical (5–20 nm) | [34] |
| CuxOy | Penicillium aurantiogriseum | Culture liquid | CuSO4 | Spherical (89–250 nm) | [35] |
| CuxOy | Penicillium citrinum | Culture liquid | CuSO4 | Spherical (85–295 nm) | [35] |
| CuxOy | Penicillium waksmanii | Culture liquid | CuSO4 | Spherical (79–179 nm) | [35] |
| CuxOy | Pleurotus florida | Bio-mass | CuCl2 | Spherical, partially spherical, oval (22.55–60.09 nm) | [36] |
| CuSO4 | Hexagonal, partially spherical (12.82–48.86 nm) |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| α-Fe2O3 | Trichoderma harzianum | Mycelial extract | FeCl3 | Spherical (average size of 207 nm) | [37] |
| γ-Fe2O3 | Penicillium expansum | Mycelial extract | FeCl3 | Spherical (15.0–66.0 nm) | [38] |
| γ-Fe2O3/α-Fe2O3 | Alternaria alternata | Mycelial extract | Fe(NO3)3 | Cubic (average size of 9 nm) | [39] |
| Fe2O3 | Aspergillus niger | Bio-mass | FeCl3 | – | [40] |
| Fe2O3 | Fusarium incarnatum | Culture liquid | FeCl2 + FeCl3 | Spherical (average size of 30.56 nm) | [41] |
| Fe2O3 | Phialemoniopsis ocularis | Culture liquid | FeCl2 + FeCl3 | Spherical (average size of 13.13 nm) | [41] |
| Fe2O3 | Penicillium pimiteouiense | Culture liquid | FeCl2 + FeCl3 | Spherical (2–16 nm) | [42] |
| Fe2O3 | Trichoderma asperellum | Culture liquid | FeCl2 + FeCl3 | Spherical (average size of 25 nm) | [41] |
| Fe3O4 | Aspergillus niger | Mycelial extract | FeCl3 | Nanoflakes (20–40 nm) | [43] |
| Fe3O4 | Aspergillus terreus | Culture liquid | Fe(NO3)3 | Spherical | [28,29] |
| Fe3O4 | Aspergillus tamarii | Culture liquid | FeSO4 + FeCl3 | Spherical (5–22 nm) | [44] |
| Fe3O4 | Fusarium oxysporum | Bio-mass | K3[Fe(CN)6] + K4[Fe(CN)6] | Quasi-spherical (20–50 nm) | [45] |
| Fe3O4 | Fusarium solani | Bio-mass | Fe2O3 | Cubic, spherical, irregular (55.3–84.2 nm) | [46] |
| Fe3O4 | Verticillium sp. | Bio-mass | K3[Fe(CN)6] + K4[Fe(CN)6] | Cubo-octahedral (100–400 nm) | [45] |
| FexOy | Amanita muscaria | Fruit body extract | FeCl2 + FeCl3 | 2.2–2.5 nm | [47] |
| FexOy | Aspergillus japonicus | Bio-mass | K3[Fe(CN)6] + K4[Fe(CN)6] | Cubic (60–70 nm) | [48] |
| FexOy | Pleurotus florida | Bio-mass | FeCl2 | Cubic (11.90–167.63 nm) | [36] |
| FeSO4 | Spherical (11.16–98.81 nm), highly agglomerated |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| TiO2 | Agaricus bisporus | Spores | Ti(OC3H7)4 | – | [54] |
| TiO2 | Aspergillus flavus | Bio-mass | TiO2 | Spherical, oval (62–74 nm) | [55] |
| TiO2 | Aspergillus flavus | Mycelial extract | TiO2 | 12–15 nm | [56] |
| TiO2 | Aspergillus niger | Mycelial extract | TiO2 salt | Spherical (73.58–106.9 nm) | [57] |
| TiO2 | Aspergillus tubingensis | Mycelial extract | TiO2 salt | Cubic, pentangular (1.5–30 nm) | [58] |
| TiO2 | Fomes fomentarius | Fruit body extract | Ti{OCH(CH3)2}4 | Irregular (80–120 nm) | [59] |
| TiO2 | Fomitopsis pinicola | Fruit body extract | Ti{OCH(CH3)2}4 | Irregular (80–120 nm) | [60] |
| TiO | Fusarium oxysporum | Bio-mass | K2TiF6 | Spherical (6–13 nm) | [61] |
| TiO2 | Humicola sp. | Bio-mass | Bulk TiO2 | Spherical (5–28 nm) | [62] |
| TiO2 | Hypsizygus ulmarius | Fruit body extract | TiCl4 | Spherical (average size of 80 nm) | [63] |
| TiO2 | Pleurotus djamor | Fruit body extract | TiCl4 | Spherical (average size of 31 nm) | [64] |
| TiO2 | Pleurotus sajor caju | Fruit body extract | TiCl4 | Spherical (average size of 85 nm) | [65] |
| TiO2 | Sachharomyces cerevisae | Living culture | TiO(OH)2 | Spherical (average size of 12.57 nm) | [66] |
| TiO2 | Sachharomyces cerevisae | Living culture | TiCl4 | Oval (10–12 nm), mesoporous | [67] |
| TiO2 | Sachharomyces cerevisae | Living culture | TiCl3 | Spherical (average size of 6.7 nm) | [68] |
| TiO2 | Tricoderma citrinoviride | Mycelial extract | Ti{OCH(CH3)2}4 | Irregular, triangular, pentagonal, spherical, rod-shaped (10–400 nm) | [69] |
| TiO2 | Trichoderma viride | Culture liquid | TiO(OH)2 | Spherical (60–86.67 nm) | [70] |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| ZnO | Acremonium potronii | Mycelial extract | Zn(CH3CO2)2 | Spherical (13–15 nm) | [74] |
| ZnO | Agarius bisporus | Fruit body extract | Zn(CH3CO2)2 | Spherical (average size of 14.48 nm) | [75] |
| ZnO | Aspergillus aeneus | Mycelial extract | Zn(CH3CO2)2 | Spherical (100–140 nm) | [76] |
| ZnO | Aspergillus fumigatus | Culture liquid | ZnSO4 | Spherical (60–80 nm) | [77] |
| ZnO | Aspergillus fumigatus | Mycelial extract | Zn(NO3)2 | Oblate spherical and hexagonal (1.2–6.8 nm) | [78] |
| ZnO | Aspergillus niger | Mycelial extract | Zn(CH3CO2)2 | Nanorods (8–38 nm) | [79] |
| ZnO | Aspergillus niger | Crushed fungal powder | ZnCl2 | Hexagonal (average size of 66 nm) | [80] |
| ZnO | Aspergillus terreus | Culture liquid | ZnSO4 | Spherical (28–63 nm) | [81] |
| ZnO | Aspergillus terreus | Mycelial extract | Zn(CH3CO2)2 | Spherical (10–45 nm) | [82] |
| ZnO | Aspergillus terreus | Culture liquid | ZnC4H6O4 | Almost spherical | [28,29] |
| ZnO | Cochliobolus geniculatus | Mycelial extract | Zn(CH3CO2)2 | Quasi-spherical (2–6 nm) | [83] |
| ZnO | Cordyceps militaris | Fruit body extract | Zn(CH3CO2)2 | Spherical, irregular (average size of 1.83 nm) | [84] |
| ZnO | Daedalea sp. | Fruit body extract | Zn(CH3CO2)2 | Irregular (average size of 14.58 nm) | [85] |
| ZnO | Fusarium keratoplasticum | Mycelial extract | Zn(CH3CO2)2 | Hexagonal (10–42 nm) | [79] |
| ZnO | Lentinula edodes | Fruit body extract | Zn(NO3)2 | Cubic, hexagonal (average size of 50 nm) | [86] |
| ZnO | Periconium sp. | Mycelial extract | Zn(NO3)2 | Quasi-spherical (16–78 nm) | [87] |
| ZnO | Pichia kudriavzevii | Fungal extract | Zn(CH3CO2)2 | Hexagonal (average size of 32 nm) | [88] |
| ZnO | Pleurotus djamor | Fruit body extract | Zn(NO3)2 | Nanorods, clusters (average size of 70–80 nm) | [64] |
| ZnO | Pleurotus florida | Bio-mass | ZnCl2 | Semi-spherical (21.27–118.36 nm) | [36] |
| ZnSO4 | Semi-spherical (9.36–58.13 nm) | ||||
| ZnO | Pleurotus floridanus | Culture liquid | Zn(NO3)2 | Spherical (average size of 34.98 nm) | [89] |
| ZnO | Pleurotus ostreatus | Fruit body extract | Zn(NO3)2 | Spherical (average size of 7.50 nm) | [90] |
| ZnO | Trichoderma harzianum | Mycelial extract | ZnSO4 | Fan- and bouquet-like structures (27–40 nm in width, 134–200 nm in length) | [33] |
| ZnO | Trichoderma harzianum | Mycelial extract | Zn(CH3CO2)2 | Spherical (average size of 30.34 nm) | [91] |
| ZnO | Trichoderma viride | Culture liquid | Zn(CH3CO2)2 | Hexagonal (average size of 63.3 nm) | [92] |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| Al2O3 | Colletotrichum sp. | Mycelial extract | AlCl3 | Spherical (average size of 30 nm) | [94] |
| AgO | Aspergillus terreus | Culture liquid | AgNO3 | Irregular spherical (60–100 nm) | [95] |
| Bi2O3 | Fusarium oxysporum | Bio-mass | Bi(NO3)3 | Quasi-spherical (5–8 nm) | [96] |
| CeO2 | Aspergillus niger | Culture liquid | CeCl3 | Spherical (5–20 nm) | [97] |
| CeO2 | Aspergillus terreus | Mycelial extract | Ce(NO3)3 | Spherical (average size of 28.5 nm) | [98] |
| CeO2 | Fusarium solani | Culture liquid | CeCl3 | Spherical (20–30 nm) | [99] |
| CeO2 | Humicola sp. | Bio-mass | Ce(NO3)3 | Spherical (12–20 nm) | [100] |
| CeO2 | Talaromyces prupureogenus | Mycelial extract | Ce(NO3)3 | Nano-sponges (average size of 21.4 nm) | [98] |
| Co3O4 | Aspergillus brasiliensis | Mycelial extract | CoSO4 | Quasi-spherical (20–27 nm) | [101] |
| Co3O4 | Aspergillus nidulans | Bio-mass | Co(C5H7O2)2 | Spherical (average size of 20.29 nm) | [102] |
| Co3O4 | Aspergillus terreus | Culture liquid | CoSO4 | Spherical | [28,29] |
| Gd2O3 | Humicola sp. | Bio-mass | GdCl3 | Quasi-spherical (3–8 nm) | [103] |
| MgO | Agaricus bisporus | Fruit body extract | Mg(CH3COO)2 | 29.6–38.6 nm | [104] |
| MgO | Aspergillus niger | Culture liquid | MgCl2 | Spherical (40–95 nm) | [105] |
| MgO | Aspergillus tubingensis | Mycelial extract | Mg(NO3)2 | Spherical (average size of 5.8 nm) | [106] |
| MgO | Trichoderma viride | Culture liquid | MgCl2 | 45.12–95.37 nm | [107] |
| MnxOy | Cladosporium halotolerans | Living culture | MnCl2 | Needle-like (2–6 nm in diameter, 0.1–1 μm in length) | [108] |
| Mn5O8 | Fusarium oxysporum | Bio-mass | (CH3CO2)2Mn· | Quasi-spherical (8–13 nm) | [109] |
| NiO | Aspergillus aculeatus | Dead bio-mass | NiCl2 | Spherical (average size of 5.89 nm) | [110] |
| NiO | Aspergillus terreus | Culture liquid | NiSO4 | Spherical | [28,29] |
| NiO | Hypocrea lixii | Dead bio-mass | NiCl2 | Average size of 3.8 nm for extracellular and 1.25 nm for intracellular NPs | [111] |
| RuO2 | Fusarium oxysporum | Bio-mass | RuCl3 | Spherical (2–5 nm) | [112] |
| Sb2O3 | Saccharomyces cerevisiae | Fungal culture | SbCl3 | Spherical (2–10 nm) | [113] |
| SeO2 | Trichoderma harzianum | Living culture | Na2SeO3 | – | [114] |
| SiO2 | Fusarium oxysporum | Bio-mass | K2SiF6 | Quasi-spherical (5–15 nm) | [61] |
| SiO2 | Fusarium oxysporum | Bio-mass | Amorphous silica present in rice husk | Quasi-spherical (2–6 nm) | [115] |
| SiO2 | Saccharomyces cervisiae | Living culture | Sodium silicate | Spherical (40–70 nm) | [116] |
| TeO2 | Mortierella humilis | Living culture | Na2TeO3 | – | [114] |
| TeO2 | Trichoderma harzianum | Living culture | Na2TeO3 | – | [114] |
| ZrO2 | Fusarium oxysporum | Bio-mass | K2ZrF6 | Quasi-spherical (3–11 nm) | [117] |
| ZrO2 | Fusarium solani | Culture liquid | zirconyl nitrate | Spherical (40–50 nm) | [118] |
| ZrO2 | Penicillium aculeatum | Culture liquid | ZrCl4 | Spherical (average size of 39.32 nm) | [119] |
| ZrO2 | Penicillium notatum | Culture liquid | ZrCl4 | Spherical (average size of 62.27 nm) | [119] |
| ZrO2 | Penicillium purpurogenome | Culture liquid | ZrCl4 | Spherical (average size of 53.60 nm) | [119] |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| α-Ag2S | Humicola sp. | Mycelial bio-mass | AgNO3, Na2SO3 | Spherical (15–40 nm) | [121] |
| Au2S | Humicola sp. | Mycelial bio-mass | HAuCl4, Na2SO3 | Spherical (20–30 nm) | [122] |
| CdS | Aspergillus niger | Mycelial bio-mass | CdCl2, Na2S | Spherical (2.7–7.5 nm) | [123] |
| CdS | Candida glabrata | Living culture | Cd(NO3)2 | – | [124] |
| CdS | Fusarium oxysporum | Living culture | CdSO4 | 5–20 nm | [125] |
| CdS | Fusarium oxysporum | Mycelial bio-mass | Cd(NO3)2, sulfur waste | Spherical (average size of 6 nm) | [126] |
| CdS | Fusarium sp. | Mycelial bio-mass | CdSO4 | Spherical (80–120 nm) | [127] |
| CdS | Phanerochaete chrysosporium | Living culture | Cd(NO3)2 | Average size of 2.56 nm | [128] |
| CdS | Pleurotus ostreatus | Mycelial bio-mass | CdSO4, Na2S | Spherical (4–5 nm) | [129] |
| CdS | Rhizopus stolonifer | Mycelial bio-mass | CdCl2, ZnS | Average size of 8.8 nm | [130] |
| CdS | Saccharomyces cerevisiae | Living culture | CdS solution | Spherical (average size of 3.57 nm) | [131] |
| CdS | Saccharomyces cerevisiae | Bio-mass | CdCl2, Na2S | Spherical (average size of 2 nm) | [132] |
| CdS | Schizosaccharo-myces pombe | Living culture | CdSO4 | Average size of 1.8 nm | [133] |
| CdS | Schizosaccharo-myces pombe | Living culture | CdSO4 | 1–1.5 nm | [134] |
| CdS | Schizosaccharo-myces pombe | Living culture | Cd(NO3)2 | – | [124] |
| CdS | Termitomyces heimii | Fruit body extract | Cd(NO3)2, Na2S | Spherical (3–5 nm) | [135] |
| CdS | Trametes versicolor | Living culture | Cd(NO3)2 | Spherical (average size of 6 nm) | [136] |
| CdS | Trichoderma harzianum | Mycelial bio-mass | CdCl2, Na2S | Spherical (3–8 nm) | [137] |
| CdS | Trichosporon jirovecii | Living culture | CdCl2 | Spherical (6–15 nm) | [138] |
| CuS | Fusarium oxysporum | Mycelial bio-mass | CuSO4 | Spherical (2–5 nm) | [139] |
| CuS | Fusarium oxysporum | Mycelial bio-mass | Copper mine wastewaters | 10–40 nm | [140] |
| PbS | Aspergillus flavus | Living culture | Pb(C2H3O2)2, Na2S | 35–100 nm | [141] |
| PbS | Rhodosporidium diobovatum | Bio-mass | Pb(NO3)2 | Spherical (2–5 nm) | [142] |
| PbS | Saccharomyces cerevisiae | Living culture | Pb(C2H3O2)2, Na2S | Spherical (0.667–6.95 nm) | [143] |
| PbS | Torulopsis sp. | Living culture | Pb(NO3)2 | 2–5 nm | [144] |
| ZnS | Agaricus bisporus | Fruit body extract | ZnCl2, Na2S | Almost spherical (2.1–3.5 nm) | [145] |
| ZnS | Aspergillus flavus | Mycelial bio-mass | ZnSO4 | Spherical (average size of 18 nm) | [146,147] |
| ZnS:Gd | Aspergillus flavus | Mycelial bio-mass | ZnSO4, Gd(NO3)2 | Spherical (10–18 nm) | [148] |
| ZnS | Aspergillus sp. | Mycelial bio-mass | ZnSO4 | Spherical (average size of 11.08 nm) | [149] |
| ZnS | Fusarium oxysporum | Mycelial bio-mass | ZnSO4 | Spherical (average size of 42 nm) | [150] |
| ZnS | Penicillium sp. | Mycelial bio-mass | ZnSO4 | Spherical (average size of 6.3 nm) | [151] |
| ZnS | Pleurotus ostreatu | Fruit body extract | ZnCl2, Na2S | Almost spherical (2.1–3.5 nm) | [152] |
| ZnS | Saccharomyces cerevisiae | Bio-mass | ZnSO4 | Spherical (30–40 nm) | [153] |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| Ag2Se | Saccharomyces cerevisiae | Living culture | AgNO3, Na2SeO3 | Average size of 3.9 nm | [157] |
| AuSe | Fusarium oxysporum | Mycelial bio-mass | HAuCl4, SeCl4 | Spherical (average size of 52 nm) | [158] |
| CdSe | Candida utilis | Living culture | CdCl2, Na2SeO3 | Average size of 4.38 nm | [159] |
| CdSe | Fusarium oxysporum | Mycelial bio-mass | CdCl2, SeCl4 | Average size of 11 nm | [160] |
| CdSe | Helminthosporum solani | Mycelial bio-mass | CdCl2, SeCl4 | Spherical, cubic (average size of 5.5 nm) | [161] |
| CdSe | Rhodotorula mucilaginosa | Bio-mass | CdCl2, Na2SeO3 | Average size of 3.2 nm | [162] |
| CdSe | Saccharomyces cerevisiae | Living culture | CdCl2, Na2SeO3 | 15–20 nm | [163] |
| CdSe | Saccharomyces cerevisiae | Living culture | CdCl2, Na2SeO3 | Average size of 2.8 nm | [164] |
| CdSe | Saccharomyces cerevisiae | Bio-mass | CdCl2, Na2SeO3 | – | [165] |
| InSe | Aspergillus niger | Living culture | InCl3, Na2SeO3 | <10 nm | [166] |
| Nd2Se3 | Fusarium oxysporum | Fungal nitrate-dependent reductase | NdCl2, SeCl4 | Spherical (average size of 18 nm) | [167] |
| PbSe | Aspergillus terreus | – | – | Nanorods (average size of 59 nm) | [168] |
| PbSe | Trichoderma sp. | Mycelial bio-mass | Pb(NO3)2, SeO2 | Cubic (10–30 nm) | [169] |
| NP | Species | Source | Precursors | Shape and Size | Reference |
|---|---|---|---|---|---|
| CdTe | Fusarium oxysporum | Mycelial bio-mass | CdCl2, TeCl4 | Spherical (15–20 nm) | [174] |
| CdTe | Rhizopus stolonifer | Mycelial bio-mass | CdCl2, TeCl4 | QDs (average size of 7.6 nm) | [133] |
| CdTe | Saccharomyces cerevisiae | Living culture | CdCl2, Na2TeO3 | QDs (2.0–3.6 nm) | [175] |
| InTe | Aspergillus niger | Living culture | InCl3, K2TeO3 | <10 nm | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A. Diversity of Mycogenic Oxide and Chalcogenide Nanoparticles: A Review. Biomimetics 2023, 8, 224. https://doi.org/10.3390/biomimetics8020224
Loshchinina EA, Vetchinkina EP, Kupryashina MA. Diversity of Mycogenic Oxide and Chalcogenide Nanoparticles: A Review. Biomimetics. 2023; 8(2):224. https://doi.org/10.3390/biomimetics8020224
Chicago/Turabian StyleLoshchinina, Ekaterina A., Elena P. Vetchinkina, and Maria A. Kupryashina. 2023. "Diversity of Mycogenic Oxide and Chalcogenide Nanoparticles: A Review" Biomimetics 8, no. 2: 224. https://doi.org/10.3390/biomimetics8020224
APA StyleLoshchinina, E. A., Vetchinkina, E. P., & Kupryashina, M. A. (2023). Diversity of Mycogenic Oxide and Chalcogenide Nanoparticles: A Review. Biomimetics, 8(2), 224. https://doi.org/10.3390/biomimetics8020224