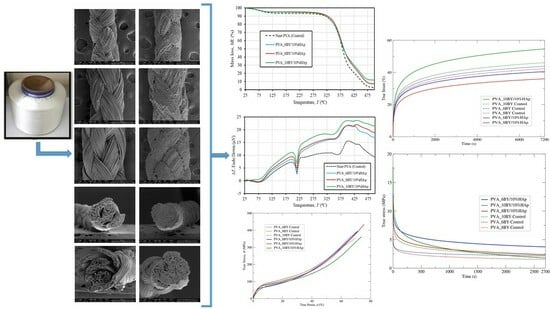
The Influence of Hydroxyapatite Crystals on the Viscoelastic Behavior of Poly(vinyl alcohol) Braid Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA/HAp Composite Braids
2.3. Morphological, Structural, Thermal, and Mechanical Characterizations
2.3.1. SEM and EDS Analyses
2.3.2. TGA
2.3.3. FTIR Spectroscopy
2.3.4. Tensile Tests
2.3.5. Creep Tensile Tests
2.3.6. Relaxation Tests
2.3.7. DMA
3. Results and Discussion
3.1. Characterisation of PVA Solutions
3.2. Selection of PVA/HAp Mixture
3.3. Experimental Tests of PVA/10%HAp Composite Braids and Pure PVA Braids
3.3.1. SEM and EDS Analyses
3.3.2. TGA Measurements
3.3.3. DTA Measurements
3.3.4. FTIR Spectroscopy Measurements
3.3.5. Monotonic Tensile Tests
3.3.6. Creep Tensile Tests
3.3.7. Relaxation Tensile Tests
3.3.8. DMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Stevens, B.; Yang, Y.; Mohandas, A.; Stucker, B.; Nguyen, K.T. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85B, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Fracture Healing Overview. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551678/ (accessed on 12 May 2021).
- Zhou, K.; He, X.; Tao, X.; Pan, F.; Yang, H. A biomechanical matched-pair comparison of two different locking plates for tibial diaphyseal comminuted fracture: Carbon fiber-reinforced poly-ether-ether-ketone (CF-PEEK) versus titanium plates. J. Orthop. Surg. Res. 2020, 15, 558. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2019, 12, 7. [Google Scholar] [CrossRef]
- Marongiu, G.; Dolci, A.; Verona, M.; Capone, A. The biology and treatment of acute long-bones diaphyseal fractures: Overview of the current options for bone healing enhancement. Bone Rep. 2020, 12, 100249. [Google Scholar] [CrossRef]
- Geiger, M. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Radius and Ulnar Shaft Fractures. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557681/ (accessed on 15 May 2021).
- Rüedi, T.P.; Arraf, J.; Babst, R.; Balogh, Z.J.; Barbosa, P.; Barla, J.D.; Baumgaertel, F.; Bernstein, B.; Blauth, M.; Borens, O.; et al. Humerus, shaft. In AO Principles of Fracture Management, 3rd ed.; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Thieme Verlagsgruppe: Stuttgart, Germany; New York, NY, USA; Delhi, India; Rio, Brazil, 2018; Volume 1, pp. 607–622. [Google Scholar]
- Rüedi, T.P.; Arraf, J.; Babst, R.; Balogh, Z.J.; Barbosa, P.; Barla, J.D.; Baumgaertel, F.; Bernstein, B.; Blauth, M.; Borens, O.; et al. Forearm, shaft. In AO Principles of Fracture Management, 3rd ed.; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Thieme Verlagsgruppe: Stuttgart, Germany; New York, NY, USA; Delhi, India; Rio, Brazil, 2018; Volume 1, pp. 657–672. [Google Scholar]
- Rüedi, T.P.; Arraf, J.; Babst, R.; Balogh, Z.J.; Barbosa, P.; Barla, J.D.; Baumgaertel, F.; Bernstein, B.; Blauth, M.; Borens, O.; et al. Biology and biomechanics in bone healing. In AO Principles of Fracture Management, 3rd ed.; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Thieme Verlagsgruppe: Stuttgart, Germany; New York, NY, USA; Delhi, India; Rio, Brazil, 2018; Volume 1, pp. 9–26. [Google Scholar]
- Lia, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, Q.; Lou, C.-W.; Lin, J.-H. Effects of ultrasonic treatment and current density on the properties of hydroxyapatite coating via electrodeposition and its in vitro biomineralization behavior. Mater. Sci. Eng. C 2019, 105, 110062. [Google Scholar] [CrossRef]
- Reith, G.; Schmitz-Greven, V.; Hensel, K.O.; Schneider, M.M.; Tinschmann, T.; Bouillon, B.; Probst, C. Metal implant removal: Benefits and drawbacks—A patient survey. BMC Surg. 2015, 15, 96. [Google Scholar] [CrossRef]
- Rüedi, T.P.; Arraf, J.; Babst, R.; Balogh, Z.J.; Barbosa, P.; Barla, J.D.; Baumgaertel, F.; Bernstein, B.; Blauth, M.; Borens, O.; et al. Implants and biotechnology. In AO Principles of Fracture Management, 3rd ed.; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Thieme Verlagsgruppe: Stuttgart, Germany; New York, NY, USA; Delhi, India; Rio, Brazil, 2018; Volume 1, pp. 27–38. [Google Scholar]
- Perren, S.M. Evolution of the internal fixation of long bone fractures: The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Ozan, S.; Munir, K.; Biesiekierski, A.; Ipek, R.; Li, Y.; Wen, C. Titanium Alloys, Including Nitinol. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 229–247. [Google Scholar]
- Rüedi, T.P.; Arraf, J.; Babst, R.; Balogh, Z.J.; Barbosa, P.; Barla, J.D.; Baumgaertel, F.; Bernstein, B.; Blauth, M.; Borens, O.; et al. Tension band principle. In AO Principles of Fracture Management, 3rd ed.; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Thieme Verlagsgruppe: Stuttgart, Germany; New York, NY, USA; Delhi, India; Rio, Brazil, 2018; Volume 1, pp. 209–216. [Google Scholar]
- Li, T.-T.; Zhang, Y.; Ling, L.; Lin, M.-C.; Wang, Y.; Wu, L.; Lin, J.-H.; Lou, C.-W. Manufacture and characteristics of HA-Electrodeposited polylactic acid/polyvinyl alcohol biodegradable braided scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 103, 103555. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, M.-C.; Chen, C.-K.; Huang, C.-L.; Chen, Y.-S.; Wen, S.-P.; Kuo, S.-T.; Lou, C.-W. Recovery evaluation of rats’ damaged tibias: Implantation of core-shell structured bone scaffolds made using hollow braids and a freeze-thawing process. Mater. Sci. Eng. C 2017, 79, 481–490. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, Y.; Chen, S.-C.; Zhai, F.-Y.; Jing, X.-K.; Wang, Y.Z. Electrospinning fabrication and characterization of poly(vinyl alcohol)/layered double hydroxides composite fibers. J. Appl. Polym. Sci. 2012, 126, 1556–1563. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Rabiee, M.; Samadikuchaksaraei, A.; Tahriri, M.; Karimi, M.; Azami, M. Influence of the value of the pH on the preparation of nano hydroxyapatite—Poly vinyl alcohol composites. J. Ceram. Process. Res. 2009, 10, 679–682. [Google Scholar]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Kuśnieruk, S.; Rogowska-Tylman, J.; Pahlevanneshan, Z.; Choińska, E.; Święszkowski, W. Fabrication and characterization of electrospun bionanocomposites of poly (vinyl alcohol)/nanohydroxyapatite/cellulose nanofibers. Int. J. Polym. Mater. Polym. 2016, 65, 660–674. [Google Scholar] [CrossRef]
- Jain, N.; Ali, S.; Singh, V.K.; Singh, K.; Bisht, N.; Chauhan, S. Creep and dynamic mechanical behavior of cross-linked polyvinyl alcohol reinforced with cotton fiber laminate composites. J. Polym. Eng. 2019, 39, 326–335. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Hu, J.; Wang, B. Porous polyetheretherketone-hydroxyapatite composite: A candidate material for orthopedic implant. Compos. Commun. 2021, 28, 100908. [Google Scholar] [CrossRef]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, Q.; Lin, J.-H.; Lou, C.-W. Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds. Nanomaterials 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Freire, T.F.; Quinaz, T.; Fertuzinhos, A.; Nguyễn, T.Q.; Moura, M.F.S.M.; Martins, M.; Zille, A.; Dourado, N. Thermal, Mechanical and Chemical Analysis of Poly(vinyl alcohol) Multifilament and Braided Yarns. Polymers 2021, 13, 3644. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D. Preparation and characterization of nano-hydroxyapatite/polyvinyl alcohol gel composites. J. Wuhan Univ. Technol. Sci. Ed. 2010, 25, 474–478. [Google Scholar] [CrossRef]
- Wu, G.; Su, B.; Zhang, W.; Wang, C. In vitro behaviors of hydroxyapatite reinforced polyvinyl alcohol hydrogel composite. Mater. Chem. Phys. 2008, 107, 364–369. [Google Scholar] [CrossRef]
- Enayati, M.S.; Neisiany, R.E.; Sajkiewicz, P.; Behzad, T.; Denis, P.; Pierini, F. Effect of nanofiller incorporation on thermomechanical and toughness of poly (vinyl alcohol)-based electrospun nanofibrous bionanocomposites. Theor. Appl. Fract. Mech. 2018, 99, 44–50. [Google Scholar] [CrossRef]
- Uma Maheshwari, S.; Samuel, V.K.; Nagiah, N. Fabrication and evaluation of (PVA/HAp/PCL) bilayer composites as potential scaffolds for bone tissue regeneration application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar] [CrossRef]
- Pearce, H.A.; Kim, Y.S.; Diaz-Gomez, L.; Mikos, A.G. Tissue Engineering Scaffolds. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1317–1334. [Google Scholar]
- Gibson, I.R. Natural and Synthetic Hydroxyapatites. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 307–317. [Google Scholar]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Łojkowski, W.; Pahlevanneshan, Z.; Ahmadi, M. Crystallinity study of electrospun poly (vinyl alcohol) nanofibers: Effect of electrospinning, filler incorporation, and heat treatment. Iran. Polym. J. 2016, 25, 647–659. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinyl alcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Ruiz-Santos, R.; Monreal-Romero, H.; Chacon-Nava, J.G. PVA/HAp composite with pork bone precursor obtained by electrospinning. Micro Nano Lett. 2017, 12, 321–324. [Google Scholar] [CrossRef]
- Rodrigues, P.J.G.; Elias, C.M.V.; Viana, B.C.; Hollanda, L.M.; Stocco, T.D.; Vasconcellos, L.M.R.; Mello, D.C.R.; Santo, F.E.P.; Marciano, F.R.; Lobo, A.O. Electrodeposition of bactericidal and bioactive nano-hydroxyapatite onto electrospun piezoelectric polyvinylidene fluoride scaffolds. J. Mater. Res. 2020, 35, 3265–3275. [Google Scholar] [CrossRef]
- Li, T.-T.; Zhang, Y.; Ren, H.-T.; Peng, H.-K.; Lou, C.-W.; Lin, J.-H. Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 260, 11776. [Google Scholar] [CrossRef] [PubMed]
- Wiria, F.E.; Chua, C.K.; Leong, K.F.; Quah, Z.Y.; Chandrasekaran, M.; Lee, M.W. Improved biocomposite development of poly(vinyl alcohol) and hydroxyapatite for tissue engineering scaffold fabrication using selective laser sintering. J. Mater. Sci. Mater. Med. 2008, 19, 989–996. [Google Scholar] [CrossRef]
- Shah, D.S.; Lawson, B.K.; Yaszemski, M. Description and Definition of Adhesives, and Related Terminology. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1181–1198. [Google Scholar]
- Hussain, R.; Tabassum, S.; Gilani, M.A.; Ahmed, E.; Sharif, A.; Manzoor, F.; Shah, A.T.; Asif, A.; Sharif, F.; Iqbal, F.; et al. In situ synthesis of mesoporous polyvinyl alcohol/hydroxyapatite composites for better biomedical coating adhesion. Appl. Surf. Sci. 2016, 364, 117–123. [Google Scholar] [CrossRef]
- Buckley, R.E. Locking plates: A current concepts review of technique and indications for use. Acta Chir. Orthop. Traumatol. Cech. 2013, 80, 185–191. [Google Scholar]
- Rüedi, T.P.; Arraf, J.; Babst, R.; Balogh, Z.J.; Barbosa, P.; Barla, J.D.; Baumgaertel, F.; Bernstein, B.; Blauth, M.; Borens, O.; et al. External fixator. In AO Principles of Fracture Management, 3rd ed.; Buckley, R.E., Moran, C.G., Apivatthakakul, T., Eds.; Thieme Verlagsgruppe: Stuttgart, Germany; New York, NY, USA; Delhi, India; Rio, Brazil, 2018; Volume 1, pp. 253–268. [Google Scholar]
- Regi, M.V.; Esbrit, P.; Salinas, A.J. Degradative Effects of the Biological Environment on Ceramic Biomaterials. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 955–971. [Google Scholar]
- Laurencin, C.T. Bone Tissue Engineering. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1373–1388. [Google Scholar]
- Jose, M.; Thomas, V.; Johson, K.; Dean, D.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Kolaja Dobra, J.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.-C.; Lin, C.-J. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Zeng, S.; Fu, S.; Guo, G.; Liang, H.; Qian, Z.; Tang, X.; Luo, F. Preparation and Characterization of Nano-Hydroxyapatite/Poly(vinyl alcohol) Composite Membranes for Guided Bone Regeneration. J. Biomed. Nanotechnol. 2011, 7, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- El-aziz, A.M.A.; El-Maghraby, A.; Taha, N.A. Comparison between polyvinyl alcohol (PVA) nanofiber and polyvinyl alcohol (PVA) nanofiber/hydroxyapatite (HA) for removal of Zn2+ ions from wastewater. Arab. J. Chem. 2017, 10, 1052–1060. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, C.-T.; Chiu, S.-J. Preparation of the PVA/HAP composite polymer membrane for alkaline DMFC application. Desalination 2008, 233, 137–146. [Google Scholar] [CrossRef]
- Satpathy, A.; Pal, A.; Sengupta, S.; Das, A.; Hasan, M.M.; Ratha, I.; Barui, A.; Bodhak, S. Bioactive Nano-Hydroxyapatite Doped Electrospun PVA-Chitosan Composite Nanofibers for Bone Tissue Engineering Applications. J. Indian Inst. Sci. 2019, 99, 289–302. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Javadpour, J.; Khavandi, A. Biomimetic synthesis and mechanical properties of hydroxyapatite/poly (vinyl alcohol) nanocomposites. Adv. Appl. Ceram. 2007, 106, 165–170. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Lou, C.W.; Kuo, S.T.; Wen, S.P.; Lin, J.H. Braided Bone Scaffolds Made by Braiding Polyvinyl Alcohol and Cross-Linked by Glutaraldehyde: Manufacturing Process and Structure Evaluation. Adv. Mater. Res. 2014, 910, 145–148. [Google Scholar] [CrossRef]
- Jamil, N.; Husin, H.; Alfida, A.W.; Aman, Z.; Hassan, Z. Characterization and Preparation of Polyvinyl Alcohol (PVA) as Inhibitor in Formation of Hydrates. Int. J. Curr. Res. Sci. Eng. Technol. 2018, 1, 578. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Cheng, Q.; Zhang, S.; Zhang, B. Preparation and properties of poly(vinyl alcohol)/silica nanocomposites derived from copolymerization of vinyl silica nanoparticles and vinyl acetate. Eur. Polym. J. 2007, 43, 1123–1131. [Google Scholar] [CrossRef]
- Campa-Siqueiros, P.; Madera-Santana, T.J.; Ayala-Zavala, J.F.; López-Cervantes, J.; Castillo-Ortega, M.M.; Herrera-Franco, P.J. Nanofibers of gelatin and polivinyl-alcohol-chitosan for wound dressing application: Fabrication and characterization. Polímeros 2020, 30, e2020006. [Google Scholar] [CrossRef]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.S.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Thornton, G.M.; Frank, C.B.; Shrive, N.G. Ligament creep behavior can be predicted from stress relaxation by incorporating fiber recruitment. J. Rheol. 2001, 45, 493–507. [Google Scholar] [CrossRef]
- Moraes, M.R.; Alves, A.C.; Toptan, F.; Martins, S.C.; Vieira, E.M.F.; Paleo, A.J.; Souto, A.P.; Santos, W.L.F.; Estevesa, M.F.; Zille, A. Glycerol/PEDOT:PSS coated woven fabric as a flexible heating element on textiles. J. Mater. Chem. C 2017, 5, 3807–3822. [Google Scholar] [CrossRef]
- Menard, K.P. Dynamic Mechanical Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Wilcox, A.G.; Buchan, K.G.; Espino, D.M. Frequency and diameter dependent viscoelastic properties of mitral valve chordae tendineae. J. Mech. Behav. Biomed. Mater. 2014, 30, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Aldred, N.; Wills, T.; Williams, D.; Clare, A. Tensile and dynamic mechanical analysis of the distal portion of mussel (Mytilus edulis) byssal threads. J. R. Soc. Interface 2007, 4, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Singh, V.K.; Chauhan, S. Dynamic and creep analysis of polyvinyl alcohol based films blended with starch and protein. J. Polym. Eng. 2018, 39, 35–47. [Google Scholar] [CrossRef]














| Braided Architectures | T Peaks of DTG (°C) [Weight Loss, WL (%)] | Residual Weight at 500 °C (wt%) | ||
|---|---|---|---|---|
| 1st Thermal Degradation | 2nd Thermal Degradation | 3rd Thermal Degradation | ||
| Neat PVA_6BY | 80 (1) [6 (6)] | 383 (1) [73 (1)] | 448 (2) [16 (7)] | 2 (9) |
| Neat PVA_8BY | 80 (3) [6 (5)] | 383 (0.3) [73 (1)] | 447 (0.5) [16 (4)] | 2 (12) |
| Neat PVA_10BY | 82 (3) [6 (5)] | 381 (0.4) [74 (1)] | 446 (2) [16 (3)] | 2 (38) |
| PVA_6BY/10%HAp | 81 (3) [5 (9)] | 379 (1) [66 (2)] | 447 (1) [19 (5)] | 7 (33) |
| PVA_8BY/10%HAp | 79 (1) [5 (8)] | 378 (1) [67 (2)] | 450 (0.4) [19 (6)] | 7 (24) |
| PVA_10BY/10%HAp | 80 (2) [5 (4)] | 374 (1) [64 (4)] | 448 (0.9) [17 (12)] | 12 (37) |
| Braided Architecture | Endothermal Peaks | ||
|---|---|---|---|
| 1st | 2nd | 3rd | |
| Tg (°C) | Tm (°C) | PVA Degradation (°C) | |
| Neat-PVA_6BY | 68 (3) | 216 (0.3) | 366 (1) |
| Neat-PVA_8BY | 68 (1) | 216 (0.3) | 366 (1) |
| Neat-PVA_10BY | 72 (8) | 215 (0.3) | 356 (3) |
| PVA_6BY/10%HAp | 77 (7) | 215 (0.3) | 353 (1) |
| PVA_8BY/10%HAp | 72 (5) | 215 (0.4) | 346 (1) |
| PVA_10BY/10%HAp | 74 (6) | 215 (1) | 344 (1) |
| Braided Architecture | Number of Filaments/Fibres | Area, A (mm2) | Young’s Modulus, E (MPa) | Ultimate Tensile Strength σu (MPa) | Ultimate Strain, εu (%) |
|---|---|---|---|---|---|
| Neat-PVA_6BY (Control) | 216 | 0.108 | 25.485 (3) | 389.741 (1) | 70.218 (0.1) |
| Neat-PVA_8BY (Control) | 288 | 0.144 | 21.126 (10) | 400.754 (5) | 70.750 (3) |
| Neat-PVA_10BY (Control) | 360 | 0.181 | 21.347 (12) | 358.021 (8) | 66.871 (9) |
| PVA_6BY/10%HAp | 216 | 0.108 | 22.621 (9) | 395.196 (5) | 71.210 (3) |
| PVA_8BY/10%HAp | 288 | 0.144 | 20.974 (10) | 433.392 (5) | 75.558 (3) |
| PVA_10BY/10%HAp | 360 | 0.181 | 14.500 (16) | 361.662 (12) | 73.694 (6) |
| Braid Architecture | Applied Stress (MPa) | Strain (%) |
|---|---|---|
| Neat-PVA_6BY (Control) | 46.77 | |
| Neat-PVA_8BY (Control) | 48.09 | |
| Neat-PVA_10BY (Control) | 42.96 | |
| PVA_6BY/10%HAp | 47.42 | |
| PVA_8BY/10%HAp | 52.01 | |
| PVA_10BY/10%HAp | 43.40 |
| Architecture | Applied Strain (%) | Stress (MPa) |
|---|---|---|
| Neat-PVA_6BY (Control) | 2.3 | |
| Neat-PVA_8BY (Control) | 2.0 | |
| Neat-PVA_10BY (Control) | 2.5 | |
| PVA_6BY/10%HAp | 2.6 | |
| PVA_8BY/10%HAp | 2.8 | |
| PVA_10BY/10%HAp | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinaz, T.; Freire, T.F.; Olmos, A.; Martins, M.; Ferreira, F.B.N.; de Moura, M.F.S.M.; Zille, A.; Nguyễn, Q.; Xavier, J.; Dourado, N. The Influence of Hydroxyapatite Crystals on the Viscoelastic Behavior of Poly(vinyl alcohol) Braid Systems. Biomimetics 2024, 9, 93. https://doi.org/10.3390/biomimetics9020093
Quinaz T, Freire TF, Olmos A, Martins M, Ferreira FBN, de Moura MFSM, Zille A, Nguyễn Q, Xavier J, Dourado N. The Influence of Hydroxyapatite Crystals on the Viscoelastic Behavior of Poly(vinyl alcohol) Braid Systems. Biomimetics. 2024; 9(2):93. https://doi.org/10.3390/biomimetics9020093
Chicago/Turabian StyleQuinaz, Tiago, Tânia F. Freire, Andrea Olmos, Marcos Martins, Fernando B. N. Ferreira, Marcelo F. S. M. de Moura, Andrea Zille, Quyền Nguyễn, José Xavier, and Nuno Dourado. 2024. "The Influence of Hydroxyapatite Crystals on the Viscoelastic Behavior of Poly(vinyl alcohol) Braid Systems" Biomimetics 9, no. 2: 93. https://doi.org/10.3390/biomimetics9020093
APA StyleQuinaz, T., Freire, T. F., Olmos, A., Martins, M., Ferreira, F. B. N., de Moura, M. F. S. M., Zille, A., Nguyễn, Q., Xavier, J., & Dourado, N. (2024). The Influence of Hydroxyapatite Crystals on the Viscoelastic Behavior of Poly(vinyl alcohol) Braid Systems. Biomimetics, 9(2), 93. https://doi.org/10.3390/biomimetics9020093