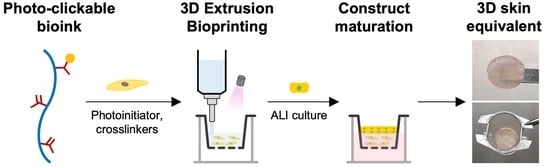
Bioinspired and Photo-Clickable Thiol-Ene Bioinks for the Extrusion Bioprinting of Mechanically Tunable 3D Skin Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Synthesis
2.2. Cell Culture
2.3. Bioink Preparation and Photocrosslinking
2.4. Rheological Measurements and Mechanical Testing
2.5. Bioprinting of 3D Skin Equivalents
2.6. Characterization of Cell Response in 3D Hydrogels
2.7. Immunohistochemistry Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Rational Design of Dermal ECM-Inspired Bioinks
3.2. Fabrication of Mechanically Tunable Dermal-like Hydrogels via Chain- and Step-Growth Mechanisms
3.3. Bioprinted Dermal Equivalents with Tunable Mechanical Properties Modulate Site-Specific Responses of Dermal Fibroblasts in 3D
3.4. Bioprinting 3D Bilayer Skin Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bayat, A.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanuf. Rev. 2017, 2, 1. [Google Scholar] [CrossRef]
- Kim, B.S.; Ahn, M.; Cho, W.-W.; Gao, G.; Jang, J.; Cho, D.-W. Engineering of diseased human skin equivalent using 3D cell printing for representing pathophysiological hallmarks of type 2 diabetes in vitro. Biomaterials 2021, 272, 120776. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part. A 2020, 26, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horiz. 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, W.-W.; Park, W.; Lee, J.-S.; Choi, M.-J.; Gao, Q.; Gao, G.; Cho, D.-W.; Kim, B.S. 3D biofabrication of diseased human skin models in vitro. Biomater. Res. 2023, 27, 80. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Dudaryeva, O.; Garciamendez-Mijares, C.E.; Kirkpatrick, B.E.; Rizzo, R.; Schimelman, J.; Anseth, K.S.; Chen, S.C.; Zenobi-Wong, M.; Zhang, Y.S. Light-based vat-polymerization bioprinting. Nat. Rev. Methods Primers 2023, 3, 47. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Byoung Soo, K.; Jung-Seob, L.; Ge, G.; Dong-Woo, C. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar]
- Habib, M.A.; Khoda, B. Rheological Analysis of Bio-ink for 3D Bio-printing Processes. J. Manuf. Process 2022, 76, 708–718. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, e1801019. [Google Scholar] [CrossRef]
- Admane, P.; Gupta, A.C.; Jois, P.; Roy, S.; Chandrasekharan Lakshmanan, C.; Kalsi, G.; Bandyopadhyay, B.; Ghosh, S. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 2019, 15, e00051. [Google Scholar] [CrossRef]
- Pereira, R.F.; Lourenco, B.N.; Bartolo, P.J.; Granja, P.L. Bioprinting a Multifunctional Bioink to Engineer Clickable 3D Cellular Niches with Tunable Matrix Microenvironmental Cues. Adv. Healthc. Mater. 2021, 10, e2001176. [Google Scholar] [CrossRef]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Murphy, S.V.; Crowell, K.; Mack, D.; Atala, A.; Soker, S. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1986–2000. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part. C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part. A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, S.; Lu, K.W.; Christie, O.; Gozelski, M.T.; Cottone, M.C.; Cottone, P.; Kianian, S.; Feng, K.-C.; Simon, M.; et al. Engineering functional skin constructs: A quantitative comparison of three-dimensional bioprinting with traditional methods. Exp. Dermatol. 2022, 31, 516–527. [Google Scholar] [CrossRef]
- Huang, S.; Yao, B.; Xie, J.; Fu, X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016, 32, 170–177. [Google Scholar] [CrossRef]
- Veiga, A.; Silva, I.V.; Duarte, M.M.; Oliveira, A.L. Current Trends on Protein Driven Bioinks for 3D Printing. Pharmaceutics 2021, 13, 1444. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Bartolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cui, S.; Song, X.; Rickel, A.P.; Sanyour, H.J.; Zheng, J.; Hu, J.; Hong, Z.; Zhou, Y.; Liu, Y. Gelatin-crosslinked pectin nanofiber mats allowing cell infiltration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110941. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Hubbell, J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845. [Google Scholar] [CrossRef] [PubMed]
- Tandara, A.A.; Mustoe, T.A. MMP- and TIMP-secretion by human cutaneous keratinocytes and fibroblasts--impact of coculture and hydration. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 108–116. [Google Scholar] [CrossRef]
- Jun, H.W.; West, J.L. Endothelialization of microporous YIGSR/PEG-modified polyurethaneurea. Tissue Eng. 2005, 11, 1133–1140. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Patterson, J.; Hubbell, J.A. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials 2011, 32, 1301–1310. [Google Scholar] [CrossRef]
- Hao, Y.; Shih, H.; Munoz, Z.; Kemp, A.; Lin, C.C. Visible light cured thiol-vinyl hydrogels with tunable degradation for 3D cell culture. Acta Biomater. 2014, 10, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Lin, C.C. Cross-linking and degradation of step-growth hydrogels formed by thiol-ene photoclick chemistry. Biomacromolecules 2012, 13, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Guagliano, G.; Volpini, C.; Camilletti, J.; Donnaloja, F.; Briatico-Vangosa, F.; Visai, L.; Petrini, P. Internally crosslinked alginate-based bioinks for the fabrication ofin vitrohepatic tissue models. Biofabrication 2023, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a Cell-Laden Conductive Hydrogel Composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bartolo, P.J. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015, 132, 42458. [Google Scholar] [CrossRef]
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol-ene alginate hydrogels as versatile bioinks for bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef]
- Mubarok, W.; Elvitigala, K.C.M.L.; Kotani, T.; Sakai, S. Visible light photocrosslinking of sugar beet pectin for 3D bioprinting applications. Carbohydr. Polym. 2023, 316, 121026. [Google Scholar] [CrossRef]
- Ahmadian, M.; Khoshfetrat, A.B.; Khatami, N.; Morshedloo, F.; Rahbarghazi, R.; Hassani, A.; Kiani, S. Influence of gelatin and collagen incorporation on peroxidase-mediated injectable pectin-based hydrogel and bioactivity of fibroblasts. J. Biomater. Appl. 2021, 36, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Schwartz, M.P.; Halevi, A.E.; Nuttelman, C.R.; Bowman, C.N.; Anseth, K.S. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater. 2009, 21, 5005–5010. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bártolo, P.J. 3D Photo-Fabrication for Tissue Engineering and Drug Delivery. Engineering 2015, 1, 90–112. [Google Scholar] [CrossRef]
- Salinas, C.N.; Anseth, K.S. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules 2008, 41, 6019–6026. [Google Scholar] [CrossRef]
- Nystrom, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Shutova, M.S.; Boehncke, W.H. Mechanotransduction in Skin Inflammation. Cells 2022, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Dongzhen, Z.; Xiaoli, C.; Jirigala, E.; Wei, S.; Zhao, L.; Tian, H.; Ping, Z.; Jianjun, L.; Yuzhen, W.; et al. Modeling human hypertrophic scars with 3D preformed cellular aggregates bioprinting. Bioact. Mater. 2022, 10, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Li, Y.; Zheng, Z.; Guan, H.; Wang, H.; Tao, K.; Liu, J.; Wang, Y.; Zhang, W.; et al. Glucocorticoid counteracts cellular mechanoresponses by LINC01569-dependent glucocorticoid receptor-mediated mRNA decay. Sci. Adv. 2021, 7, eabd9923. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Perepelyuk, M.; Soulas, E.M.; Lee, G.Y.; Wells, R.G.; Burdick, J.A. Gradually softening hydrogels for modeling hepatic stellate cell behavior during fibrosis regression. Integr. Biol. 2016, 8, 720–728. [Google Scholar] [CrossRef]
- Hewawasam, R.S.; Blomberg, R.; Serbedzija, P.; Magin, C.M. Chemical Modification of Human Decellularized Extracellular Matrix for Incorporation into Phototunable Hybrid-Hydrogel Models of Tissue Fibrosis. ACS Appl. Mater. Interfaces 2023, 15, 15071–15083. [Google Scholar] [CrossRef]
- Holt, B.; Tripathi, A.; Morgan, J. Viscoelastic response of human skin to low magnitude physiologically relevant shear. J. Biomech. 2008, 41, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked Collagen Hydrogel Matrix Resisting Contraction To Facilitate Full-Thickness Skin Equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, M.; Jia, Y.B.; Niu, L.L.; Huang, G.Y.; Xu, F. Control of fibroblast shape in sequentially formed 3D hybrid hydrogels regulates cellular responses to microenvironmental cues. Npg Asia Mater. 2020, 12, 45. [Google Scholar] [CrossRef]
- Bott, K.; Upton, Z.; Schrobback, K.; Ehrbar, M.; Hubbell, J.A.; Lutolf, M.P.; Rizzi, S.C. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 2010, 31, 8454–8464. [Google Scholar] [CrossRef] [PubMed]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Evaluation of in vitro human skin models for studying effects of external stressors and stimuli and developing treatment modalities. View 2022, 3, 20210012. [Google Scholar] [CrossRef]
- Loewa, A.; Feng, J.J.; Hedtrich, S. Human disease models in drug development. Nat. Rev. Bioeng. 2023, 1, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Rahman-Zaman, A.; VanderBurgh, J.A.; Taufalele, P.V.; Jain, A.; Erickson, D.; Bordeleau, F.; Reinhart-King, C.A. Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat. Commun. 2019, 10, 4185. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-p.; Stowers, R.; Chaudhuri, O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 2019, 10, 529. [Google Scholar] [CrossRef]
- Caliari, S.R.; Vega, S.L.; Kwon, M.; Soulas, E.M.; Burdick, J.A. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials 2016, 103, 314–323. [Google Scholar] [CrossRef]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebiano, L.B.; Presa, R.; Vieira, F.; Lourenço, B.N.; Pereira, R.F. Bioinspired and Photo-Clickable Thiol-Ene Bioinks for the Extrusion Bioprinting of Mechanically Tunable 3D Skin Models. Biomimetics 2024, 9, 228. https://doi.org/10.3390/biomimetics9040228
Bebiano LB, Presa R, Vieira F, Lourenço BN, Pereira RF. Bioinspired and Photo-Clickable Thiol-Ene Bioinks for the Extrusion Bioprinting of Mechanically Tunable 3D Skin Models. Biomimetics. 2024; 9(4):228. https://doi.org/10.3390/biomimetics9040228
Chicago/Turabian StyleBebiano, Luís B., Rafaela Presa, Francisca Vieira, Bianca N. Lourenço, and Rúben F. Pereira. 2024. "Bioinspired and Photo-Clickable Thiol-Ene Bioinks for the Extrusion Bioprinting of Mechanically Tunable 3D Skin Models" Biomimetics 9, no. 4: 228. https://doi.org/10.3390/biomimetics9040228
APA StyleBebiano, L. B., Presa, R., Vieira, F., Lourenço, B. N., & Pereira, R. F. (2024). Bioinspired and Photo-Clickable Thiol-Ene Bioinks for the Extrusion Bioprinting of Mechanically Tunable 3D Skin Models. Biomimetics, 9(4), 228. https://doi.org/10.3390/biomimetics9040228