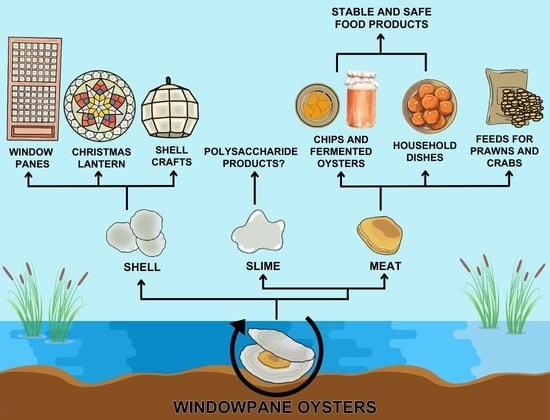
History and Prospects for the Sustainability and Circularity of the Windowpane Oyster Placuna placenta Fishery in the Philippines
Abstract
:1. Introduction
2. Natural History of Placuna placenta
3. Exploitation and Benefits Derived from Bivalves
3.1. Historical Perspective of Exploitation
3.2. Modern Economic Exploitation
3.3. Nutritional Benefits of Bivalves
| Bivalves | Vitamin A (retinol), µg | Thiamin mg | Riboflavin mg | Niacin mg | Na mg | Ca mg | P mg | Fe mg | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Placuna placenta | 95 | 0.02 | 0.11 | 1.4 | 467 | 110 | 257 | 17.3 | [34] |
| Magallana bilineata | n.d. * | n.d. | n.d. | n.d. | 159–175 | 518–530 | [39] | ||
| Crassostrea sp. | 110 | 0.21 | 0.2 | 1.7 | 882 | 147 | 77 | 5.9 | [34] |
| Perna viridis | 420 | 0.26 | 0.07 | 2.2 | 528 | 176 | 144 | 3.5 | [34] |
| Corbicula manilensis | 165 | 0 | 0.22 | 1.5 | 34 | 179 | 79 | 1.5 | [34] |
| Cypraeidae sp. | 130 | 0 | 0.16 | 1.5 | 645 | 153 | 125 | 7.3 | [34] |
| Placopecten magellanicus | n.d. | n.d. | 0.067 | 1.54 | 207 | 11 | 277 | 0.38 | [40,41] |
| Bivalves | Saturated Fatty Acids, g | Monounsaturated Fatty Acds, g | Polyunsaturated Fatty Acids, g | Cholesterol mg | Reference |
|---|---|---|---|---|---|
| Placuna placenta | 0.37 | 0.19 | 0.50 | 29 | [34] |
| Magallana bilineata | 0.48–0.61 | 0.13–0.22 | 0.27–0.36 | 31–35 | [39] |
| Crassostrea sp. | 0.44 | 0.23 | 0.61 | 35 | [34] |
| Perna viridis | 1.42 | 1.70 | 2.03 | 74.2 | [34,39] |
| Corbicula manilensis | 0.33 | 0.19 | 0.26 | 84 | [34] |
| Cypraeidae sp. | 0.14 | 0.09 | 0.14 | 22 | [34] |
| Placopecten magellanicus | 0.28 | 0.13–0.14 | 0.35–0.37 | 36.7 | [34,43] |
3.4. Pathogens, Allergens, and Contaminants in Bivalves
4. Potentials/Processing Possibilities for P. placenta By-Products
4.1. Bioactive Compounds in Marine Invertebrates
4.1.1. Peptides
4.1.2. Shell Proteins and Polysaccharides
4.1.3. Glycosaminoglycans in Marine Invertebrates
4.2. P. placenta Oyster By-Products and Waste
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022; Toward Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- PHILFIS. Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources: Quezon City, Philippines, 2021. Available online: https://www.bfar.da.gov.ph/wp-content/uploads/2022/11/2021-Fisheries-Profile-FINAL-FILE.pdf (accessed on 14 September 2023).
- Adan, R.I.Y. SEAFDEC Asian Aquaculture; Aquaculture Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2000; pp. 23, 31. Available online: https://repository.seafdec.org.ph/handle/10862/2739 (accessed on 1 August 2023).
- Dame, R.F.; Kenneth, M.J. Ecology of Marine Bivalves an Ecosystem Approach, 2nd ed.; Oven, A., Ed.; Marine Science; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2012; ISBN 9781439839096. [Google Scholar]
- Vaughn, C.C.; Hoellein, T.J. Bivalve Impacts in Freshwater and Marine Ecosystems. Annu. Rev. 2018, 49, 183–208. [Google Scholar] [CrossRef]
- Hornell, J. Marine Zoology of Okhamandal India; Biodiversity Heritage Library: Washington, DC, USA, 1909; Available online: https://www.biodiversitylibrary.org/item/15808#page/46/mode/1up. (accessed on 22 August 2023).
- Jiratipayabood, P. Windowpane Oysters (Placuna placenta) in Siit Bay; Marine Conservation Philippines: Zamboanguita, Philippines, 2017; pp. 1–11. Available online: https://www.marineconservationphilippines.org/wp-content/uploads/2018/02/windowpane-oyster-report.pdf (accessed on 1 August 2023).
- Li, L.; Ortiz, C. Pervasive Nanoscale Deformation Twinning as a Catalyst for Efficient Energy Dissipation in a Bioceramic Armour. Nat. Mater. 2014, 13, 501–507. [Google Scholar] [CrossRef]
- Yonge, M. Form and Evolution in the Anomiacea (Mollusca: Bivalvia)-Pododesmus, Anomia, Patro, Enigmonia (Anomiidae): Placunanomia, Placuna (Placunidae Fam. Nov.). Philos. Trans. R. Soc. B 1977, 276, 453–523. [Google Scholar]
- Madrones-Ladja, J.A. Salinity Effect on the Embryonic Development, Larval Growth and Survival at Metamorphosis of Placuna Placenta Linnaeus (1758). Aquaculture 2002, 214, 411–418. [Google Scholar] [CrossRef]
- Gifford, S.; O’Connor, W.; Koller, C.E.; MacFarlane, G.R.; Dunstan, R.H. Aquatic Zooremediation: Deploying Animals to Remediate Contaminated Aquatic Environments. Trends Biotechnol. 2007, 25, 60–65. [Google Scholar] [CrossRef]
- O’Connor, T.P. National Distribution of Chemical Concentrations in Mussels and Oysters in the USA. Mar. Environ. Res. 2002, 53, 117–143. [Google Scholar] [CrossRef]
- Mahdi, W. The Dispersal of Austronesian Boat Forms in the Indian Ocean. In One World Archaeology; Roger, B., Matthew, S., Eds.; Archaeology and Language III: Artefacts Languages, and Texts; Routledge: London, UK; New York, NY, USA, 1999; Volume 34, pp. 144–179. ISBN 978-0-415-10054-0. [Google Scholar]
- Bellina, B. Southeast Asia and the Early Maritime Silk Road. In Lost Kingdoms: Hindu-Buddhist Sculpture of Early Southeast Asia; Guy, J., Ed.; Yale University Press: New York, NY, USA, 2014; pp. 22–25. ISBN 978-1-58839-524-5. [Google Scholar]
- National Commission on Indigenous peoples Badjao. The Indigenous Peoples of the Philippines; Rex Bookstore Inc.: Quezon City, Philippines, 2007; pp. 3–5. ISBN 978-971-23-4670-5. [Google Scholar]
- De Noceda, J.J.; De Sanlucar, P. Vocabulario de la Lengua Tagala: Compuesto por Varios Religiosos Doctos y Graves, y Coordinado; Ramirez y Giraudier: Manila, Philippines, 1860; Available online: https://books.google.co.uk/books?id=PTIOAAAAIAAJ&printsec=frontcover#v=onepage&q&f=false (accessed on 26 September 2023).
- Bautista, N. What You Need to Know about Capiz Shell Jewelry. 2023. Available online: https://www.shopcambio.co/blogs/news/what-you-need-to-know-about-capiz-shell-jewelry (accessed on 1 August 2023).
- Floren, A.S. The Philippine Shell Industry with Special Focus on Mactan Cebu; Department of Environment and Natural Resources: Quezon City, Philippines, 2003; pp. 1–50. Available online: http://oneocean.org/download/db_files/philippine_shell_industry.pdf (accessed on 1 August 2023).
- Park, M. Capiz Shells and Their Uses. 2009. Available online: http://ecop.pbworks.com/w/page/18520528/Capiz%20shells%20and%20their%20uses%200809 (accessed on 1 August 2023).
- PHILFIS. Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources: Quezon City, Philippines, 2018. Available online: https://beta.bfar.da.gov.ph/wp-content/uploads/2021/05/Philippine-Fisheries-Profile-2018.pdf (accessed on 1 August 2023).
- PHILFIS. Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources: Quezon City, Philippines, 1996. Available online: https://www.bfar.da.gov.ph/wp-content/uploads/2021/05/Philippine-Fisheries-Profile-1996.pdf (accessed on 1 August 2023).
- PHILFIS. Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources: Quezon City, Philippines, 1994. Available online: https://www.bfar.da.gov.ph/wp-content/uploads/2021/05/Philippine-Fisheries-Profile-1994.pdf (accessed on 1 August 2023).
- PHILFIS. Philippine Fisheries Profile; Department of Agriculture, Bureau of Fisheries and Aquatic Resources: Quezon City, Philippines, 1991. Available online: https://www.bfar.da.gov.ph/wp-content/uploads/2021/05/Philippine-Fisheries-Profile-1991.pdf (accessed on 1 August 2023).
- Lopez, N.A. Status of Threatened Species and Stock Enhancement Activities in the Philippine Fisheries; SEAFDEC/AQD Institutional Repository: Iloilo, Philippines, 2006; Available online: http://hdl.handle.net/10862/2941 (accessed on 1 September 2023).
- Hermoso, R.S. Kapis Chips: A Nutritious Finger Food from the Sea; Bureau of Agricultural Research. 2018. Available online: https://bar.gov.ph/index.php/media-resources/news-and-events/175-kapis-chips-a-nutritious-finger-food-from-the-sea (accessed on 23 August 2023).
- Garibay, S.S.; Golez, S.N.; Unggui, A.S. Transplantation, Hatchery, and Grow-out of Window-Pane Oyster Placuna Placenta in Guimaras and Iloilo. In Research Output of the Fisheries Sector Program; Bagarinao, T.U., Ed.; Reports on Fisheries and Aquaculture; SEAFDEC/AQD-Department of Agriculture (DA)—Philippines: Iloilo, Philippines; Bureau of Agricultural Research, Department of Agriculture: Quezon City, Philippines, 2007; Volume 2, pp. 119–122. ISBN 971-8511-77-6. [Google Scholar]
- Gallardo, W.G.; De Castro, M.T.R.; Buensuceso, R.T.; Espegadera, C.C.; Baylon, C.C. Gonad Development of Placuna Placenta Linnaeus Fed Isochrysis Galbana Parke, Tetraselmis Tetrahele (G.S. West) Butch, or Their Combination. Aquaculture 1992, 102, 357–361. [Google Scholar] [CrossRef]
- Gallardo, W.G.; Siar, S.V.; EncenaI, V. Exploitation of the Window-Pane Shell Placuna Placenta in the Philippines. Biol. Conserv. 1995, 73, 33–38. [Google Scholar] [CrossRef]
- Gallardo, W.G.; Ladja, J.M.; Baliao, D.D. SEAFDEC Asian Aquaculture; Aquaculture Department of the Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2001; pp. 22–23. Available online: https://repository.seafdec.org.ph/bitstream/handle/10862/2720/SAAv23n05-06.pdf?sequence=1 (accessed on 1 August 2023).
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional Value and Food Safety of Bivalve Molluscan Shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- FDA CFR. Code of Federal Regulations Title 21. 2023. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=113.3 (accessed on 1 August 2023).
- Miletic, I.; Miric, M.; Lalic, Z.; Sobajic, S. Composition of Lipids and Proteins of Several Species of Molluscs, Marine and Terrestrial, from the Adriatic Sea and Serbia. Food Chem. 1991, 41, 303–308. [Google Scholar] [CrossRef]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef]
- Nacionales, K.B.; Rodriguez, R.G.; Avena, E.M.; David, A.L.E.; Serrano, J.D.; Baylosis, M.A.C.; Lundag, A.G. Food and Nutrition Research Institute Philippine Food Composition Table Online Database (PhilFCT); Department of Science and Technology Food and Nutrition Research Institute (DOST-FNRI) Bicutan: Taguig City, Philippines, 2019; p. 191. ISBN 978-971-8769-44-7. [Google Scholar]
- Asha, K.K.; Anandan, R.; Mathew, S.; Lakshmanan, P.T. Biochemical Profile of Oyster Crassostrea Madrasensis and Its Nutritional Attributes. Egypt. J. Aquat. Res. 2014, 40, 35–41. [Google Scholar] [CrossRef]
- Naidu, K.S.; Botta, J.R. Taste Panel Assessment and Proximate Composition of Cultured and Wild Sea Scallops, Placopecten Magellanicus (Gmelin). Aquaculture 1978, 15, 243–247. [Google Scholar] [CrossRef]
- Palanog, A.D.; Calayugan, M.I.C.; Descalsota-Empleo, G.I.; Amparado, A.; Inabangan-Asilo, M.A.; Arocena, E.C.; Cruz, P.C.S.; Borromeo, T.H.; Lalusin, A.; Hernandez, J.E.; et al. Zinc and Iron Nutrition Status in the Philippines Population and Local Soils. Front. Nutr. 2019, 6, 81. [Google Scholar] [CrossRef]
- Solon, F.S.; Popkin, B.M.; Fernandez, T.L.; Latham, M.C. Vitamin A Deficiency in the Philippines: A Study of Xerophthalmia in Cebu. Am. J. Clin. Nutr. 1978, 31, 360–368. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakkalakal, S.J.; Joseph, D.; Asokan, P.K.; Vijayan, K.K. Nutritional and Antioxidative Attributes of Green Mussel (Perna viridis L.) from the Southwestern Coast of India. J. Aquat. Food Prod. Technol. 2016, 25, 968–985. [Google Scholar] [CrossRef]
- Fisher, R.A.; Dupaul, W.D.; Rippen, T.E. Nutritional, Proximate, and Microbial Characteristics of Phosphate Processed Sea Scallops (Placopecten Magellanicus). J. Muscle Foods 1996, 7, 73–92. [Google Scholar] [CrossRef]
- Bergé, J.-P.; Barnathan, G. Fatty Acids from Lipids of Marine Organisms: Molecular Biodiversity, Roles as Biomarkers, Biologically Active Compounds, and Economical Aspects. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 96, pp. 49–125. ISBN 978-3-540-31549-0. [Google Scholar]
- Hossain, Z.; Takahashi, K. Health Benefits of Bio-Functional Marine Lipids. In Proceedings of the Second International Congress on Seafood Technology on Sustainable Innovative and Healthy Seafood, Anchorage, AK, USA, 10–13 May 2010; Ryder, J., Ed.; FAO fisheries and aquaculture proceedings. Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. ISBN 978-92-5-107108-3. [Google Scholar]
- Napolitano, G.E.; MacDonald, B.A.; Thompson, R.J.; Ackman, R.G. Lipid Composition of Eggs and Adductor Muscle in Giant Scallops (Placopecten Magellanicus) from Different Habitats. Mar. Biol. 1992, 113, 71–76. [Google Scholar] [CrossRef]
- Lattos, A.; Chaligiannis, I.; Papadopoulos, D.; Giantsis, I.A.; Petridou, E.I.; Baker, G.L.; Staikou, A.; Michaelidis, B. How Safe to Eat Are Raw Bivalves? Host Pathogenic and Public Health Concern Microbes within Mussels, Oysters, and Clams in Greek Markets. Foods 2021, 10, 2793. [Google Scholar] [CrossRef]
- Gosling, E. Marine Bivalve Mollusks, 2nd ed.; Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tedde, T.; Piras, G.; Salza, S.; Nives, R.M.; Sanna, G.; Tola, S.; Culurgioni, J.; Piras, C.; Merella, P.; Garippa, G.; et al. Investigation into Cryptosporidium and Giardia in Bivalve Mollusks Farmed in Sardinia Region and Destined for Human Consumption. Ital. J. Food Saf. 2013, 2, 91–93. [Google Scholar] [CrossRef]
- Brinkley, M. Raw Oysters and Food Poisoning. Available online: https://www.livestrong.com/article/435577-side-effects-of-eating-oysters/ (accessed on 1 August 2023).
- Pagador, E.G. Parasites of Window-Pane Oyster (Placuna Placenta Linnaeus, 1758) from Trapiche, Oton in West Central Philippines. Philipp. Agric. Sci. 2015, 98, 323–327. [Google Scholar]
- FARRP Allergenic Foods and Their Allergens: Molluscan Shellfish. Available online: https://farrp.unl.edu/informallmollshellfish (accessed on 23 August 2023).
- Taylor, S.L. Chapter 4—Molluscan Shellfish Allergy. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 54, pp. 139–177. [Google Scholar]
- Huan, F.; Han, T.-J.; Liu, M.; Li, M.-S.; Yang, Y.; Liu, Q.-M.; Lai, D.; Cao, M.-J.; Liu, G.-M. Identification and Characterization of Crassostrea angulata Arginine Kinase, a Novel Allergen That Causes Cross-Reactivity among Shellfish. Food Funct. 2021, 12, 9866–9879. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. BioMed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 80, pp. 209–276. [Google Scholar]
- Latire, T.; Legendre, F.; Bigot, N.; Carduner, L.; Kellouche, S.; Bouyoucef, M.; Carreiras, F.; Marin, F.; Lebel, J.-M.; Galéra, P.; et al. Shell Extracts from the Marine Bivalve Pecten Maximus Regulate the Synthesis of Extracellular Matrix in Primary Cultured Human Skin Fibroblasts. PLoS ONE 2014, 9, e99931. [Google Scholar] [CrossRef]
- Hedlund, K.D.; Coyne, D.P.; Sanford, D.M.; Huddelson, J. The Heparin Recall of 2008. Perfusion 2012, 28, 61–65. [Google Scholar] [CrossRef]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. The Heparanome and Regulation of Cell Function: Structures, Functions and Challenges. Front. Biosci. 2008, 13, 4309–4338. [Google Scholar] [CrossRef]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. A Systems Biology Approach for the Investigation of the Heparin/Heparan Sulfate Interactome. J. Biol. Chem. 2011, 286, 19892–19904. [Google Scholar] [CrossRef]
- Woznica, A.; Gerdt, J.P.; Hulett, R.E.; King, N. Mating in the Closest Living Relatives of Animals Is Induced by a Bacterial Chondroitinase. Cell 2017, 170, 1175–1183.e11. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K.; Ozbek, S. Evolution of Glycosaminoglycans: Comparative Biochemical Study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef]
- Volpi, N.; Maccari, F. Glycosaminoglycan Composition of the Large Freshwater Mollusc Bivalve Anodonta Anodonta. Biomacromolecules 2005, 6, 3174–3180. [Google Scholar] [CrossRef]
- Brito, A.S.; Arimatéia, D.S.; Souza, L.R.; Lima, M.A.; Santos, V.O.; Medeiros, V.P.; Ferreira, P.A.; Silva, R.A.; Ferreira, C.V.; Justo, G.Z.; et al. Anti-Inflammatory Properties of a Heparin-like Glycosaminoglycan with Reduced Anti-Coagulant Activity Isolated from a Marine Shrimp. Bioorg. Med. Chem. 2008, 16, 9588–9595. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.S.; Cavalcante, R.S.; Palhares, L.C.G.F.; Hughes, A.J.; Andrade, G.P.V.; Yates, E.A.; Nader, H.B.; Lima, M.A.; Chavante, S.F. A Non-Hemorrhagic Hybrid Heparin/Heparan Sulfate with Anticoagulant Potential. Carbohydr. Polym. 2014, 99, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Hughes, A.J.; Veraldi, N.; Rudd, T.R.; Hussain, R.; Brito, A.S.; Chavante, S.F.; Tersariol, I.I.; Siligardi, G.; Nader, H.B.; et al. Antithrombin Stabilisation by Sulfated Carbohydrates Correlates with Anticoagulant Activity. Med. Chem. Commun. 2013, 4, 870. [Google Scholar] [CrossRef]
- Chavante, S.F.; Brito, A.S.; Lima, M.; Yates, E.; Nader, H.; Guerrini, M.; Torri, G.; Bisio, A. A Heparin-like Glycosaminoglycan from Shrimp Containing High Levels of 3-O-Sulfated D-Glucosamine Groups in an Unusual Trisaccharide Sequence. Carbohydr. Res. 2014, 390, 59–66. [Google Scholar] [CrossRef]
- Dreyfuss, J.L.; Regatieri, C.V.; Lima, M.A.; Paredes-gamero, E.J.; Brito, A.S.; Chavante, S.F.; Belfort, R., Jr.; Farah, M.E.; Nader, H.B. A Heparin Mimetic Isolated from a Marine Shrimp Suppresses Neovascularization. J. Thromb. Haemost. 2010, 8, 1828–1837. [Google Scholar] [CrossRef]
- Pavão, M.S.G. Glycosaminoglycans Analogs from Marine Invertebrates: Structure, Biological Effects, and Potential as New Therapeutics. Front. Cell. Infect. Microbiol. 2014, 4, 123. [Google Scholar] [CrossRef]
- Karamanou, K.; Espinosa, D.C.R.; Fortuna-Costa, A.; Pavão, M.S.G. Biological Function of Unique Sulfated Glycosaminoglycans in Primitive Chordates. Glycoconj. J. 2017, 34, 277–283. [Google Scholar] [CrossRef]
- Gonzaga, R. After Jessica Sanchez, Samal’s Capiz Products Now in Limelight. Philippine Daily Inquirer. 31 May 2012. Available online: https://business.inquirer.net/62617/after-jessica-sanchez-samals-capiz-products-now-in-limelight (accessed on 1 August 2023).
- Brion, A.C. Kapis Chips: New Delicacy from the Sea. The Philippine Star. 19 July 2015, p. B-4. Available online: https://www.pressreader.com/philippines/the-philippine-star/20150719/282076275555753 (accessed on 1 August 2023).



| Bivalve | Scientific Name | Moisture % | Ash % | Protein % | Fat % | Carbohydrate% | Ref. |
|---|---|---|---|---|---|---|---|
| Windowpane oyster | Placuna placenta | 70.2 | 1.8 | 23.3 | 1.4 | 3.3 | [34] |
| Philippine Slipper oyster | Magallana bilineata | 82.64 | 1.01 | 9.41 | 3.25 | 3.2 | [35] |
| Oyster | Crassostrea sp. | 85.5 | 1.7 | 5.9 | 1.7 | 5.2 | [34] |
| Mussel, green | Perna viridis | 64.2 | 3.6 | 13.6 | 7.5 | 11.1 | [34] |
| Clam, freshwater | Corbicula manilensis | 78 | 0.7 | 7.6 | 1.9 | 11.8 | [34] |
| Clam | Cypraeidae sp. | 87.2 | 1.9 | 6.7 | 0.7 | 3.5 | |
| Sea scallop | Placopecten magellanicus | 78.50 | 1.6 | 17.4 | 0.48 | 1.99 | [34,36] |
| Bacteria | Viruses | Parasites | Reference |
|---|---|---|---|
| Pathogenic to humans | |||
| Salmonella enteritidis | hepatitis A virus | Echinocephalus sinensis | [33,45] |
| Salmonella paratyphi | norovirus | Sulcascaris sulcata | [33,45] |
| Salmonella typhimurium | calicivirus | Giardia | [33,46] |
| Shigella spp. | astrovirus | Cryptosporidium | [33,46] |
| E. coli serotype O157:H7 | - | Toxoplasma | [33,46] |
| Campylobacter spp. | - | - | [33] |
| Vibrio spp. | - | - | [33] |
| V. parahemolyticus | - | - | [47] |
| Aeromonas spp. | - | - | [33] |
| Plesiomonas spp. | - | - | [33] |
| Yersinia enterocolitica | - | - | [33] |
| Clostridium botulinum | - | - | [33] |
| Listeria monocytogenes | - | - | [33] |
| Not pathogenic to humans | |||
| - | - | pea crabs (Pinnotheres sp.) | [48] |
| - | - | Tylocephalum sp. | [48] |
| - | - | Bucephalus sp. | [48] |
| - | - | Ancistroma sp. | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustia, J.M.; Antonino, J.P.; Velasco, R.R.; Lima, M.A.; Yates, E.A.; Fernig, D.G. History and Prospects for the Sustainability and Circularity of the Windowpane Oyster Placuna placenta Fishery in the Philippines. Fishes 2023, 8, 493. https://doi.org/10.3390/fishes8100493
Rustia JM, Antonino JP, Velasco RR, Lima MA, Yates EA, Fernig DG. History and Prospects for the Sustainability and Circularity of the Windowpane Oyster Placuna placenta Fishery in the Philippines. Fishes. 2023; 8(10):493. https://doi.org/10.3390/fishes8100493
Chicago/Turabian StyleRustia, Jessica M., Judith P. Antonino, Ravelina R. Velasco, Marcelo A. Lima, Edwin A. Yates, and David G. Fernig. 2023. "History and Prospects for the Sustainability and Circularity of the Windowpane Oyster Placuna placenta Fishery in the Philippines" Fishes 8, no. 10: 493. https://doi.org/10.3390/fishes8100493
APA StyleRustia, J. M., Antonino, J. P., Velasco, R. R., Lima, M. A., Yates, E. A., & Fernig, D. G. (2023). History and Prospects for the Sustainability and Circularity of the Windowpane Oyster Placuna placenta Fishery in the Philippines. Fishes, 8(10), 493. https://doi.org/10.3390/fishes8100493