Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles
Abstract
:1. Introduction
2. Materials and Methods
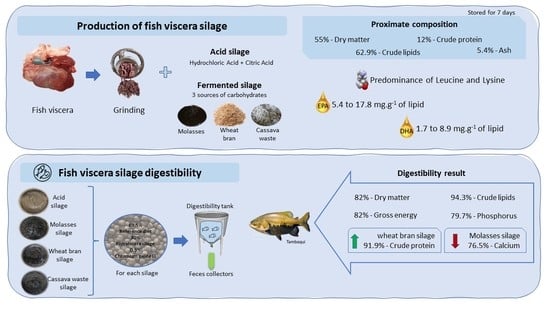
2.1. Production of Fish Viscera Silage
2.2. Composition of Amino Acids
2.3. Composition of Fatty Acids
2.4. Determination of the Apparent Digestibility Coefficient (ADC)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Fishery and Aquaculture Country Profiles—Mexico. In Country Profile Fact Sheets; Fisheries and Aquaculture Division: Rome, Italy, 2022; Available online: https://www.fao.org/fishery/en/facp/mex?lang=en (accessed on 8 November 2022).
- Mozumder, M.M.H.; Uddin, M.M.; Schneider, P.; Raiyan, H.I.; Trisha, M.G.A.; Tahsin, T.H.; Newase, S. Sustainable Utilization of Fishery Waste in Bangladesh—A Qualitative Study for a Circular Bioeconomy Initiative. Fishes 2022, 7, 84. [Google Scholar] [CrossRef]
- Toppe, J.; Olsen, R.L.; Peñarubia, O.R.; James, D.G. Production and Utilization of Fish Silage. A Manual on How to Turn Fish Waste into Profit and a Valuable Feed Ingredient or Fertilizer; FAO: Rome, Italy, 2018; Available online: www.fao.org/3/i9606en/I9606EN.pdf (accessed on 7 November 2022).
- Kuley, E.; Özyurt, G.; Özogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Özogul, F. The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-Based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability. Microorganisms 2020, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, R.M.; Viegas, E.M.M.; Carneiro, D.J. Amino acid composition of processed fish silage using different raw materials. Anim. Feed Sci. Technol. 2003, 105, 199–204. [Google Scholar] [CrossRef]
- Banze, J.F.; da Silva, M.F.O.; Enke, D.B.S.; Fracalossi, D.M. Acid silage of tuna viscera: Production, composition, quality and digestibility. Bol. Inst. Pesca 2017, 44, 24–34. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; García-Cano, I.; Dabrowski, K.; Jiménez-Flores, R. Bacterial Diversity Analysis and Evaluation Proteins Hydrolysis During the Acid Whey and Fish Waste Fermentation. Microorganisms 2021, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Afreen, M.; Ucak, I. Fish processing wastes used as feed ingredient for animal feed and aquaculture feed. Surv. Fish. Sci. 2020, 6, 55–64. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). 2021. Available online: https://sidra.ibge.gov.br/Tabela/3940 (accessed on 29 November 2022).
- Woynárovich, A.; Van Anrooy, R. Field Guide to the Culture of Tambaqui (Colossoma macropomum, Cuvier, 1816); FAO Fisheries and Aquaculture Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; p. I-121. [Google Scholar]
- Hilsdorf, A.W.S.; Hallerman, E.; Valladão, G.M.R.; Zaminhan-Hassemer, M.; Hashimoto, D.T.; Dairiki, J.K.; Takahashi, L.S.; Albergaria, F.C.; Gomes, M.E.D.S.; Venturieri, R.L.L.; et al. The farming and husbandry of Colossoma macropomum: From Amazonian waters to sustainable production. Rev. Aquac. 2022, 14, 993–1027. [Google Scholar] [CrossRef]
- Marcos, R.; Filho, R.A.C.; De Abreu, J.S.; Seraphim, G.N.; Silva, A.C.; Fornari, D.C.; Ribeiro, R.P.; Ferreira, Y.A.; Gama, K.F.; Povh, J.A. Weight and body yield of selectively bred tambaqui (Colossoma macropomum) farmed in different environments. An. Acad. Bras. Ciênc. 2020, 92, e20180675. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC International, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2005; Volume 2. [Google Scholar]
- Spies, J.R. Determination of tryptophan in proteins. Anal. Chem. 1967, 39, 1412–1416. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Júnior, O.O.S.; Montanher, P.F.; Bonafe, E.G.; Prado, I.N.D.; Maruyama, S.A.; Matsushita, M.; Visentainer, J.V. A Simple, Fast and Efficient Method for Transesterification of Fatty Acids in Foods Assisted by Ultrasound Energy. J. Braz. Chem. Soc. 2014, 25, 1712–1719. [Google Scholar] [CrossRef]
- Joseph, J.D.; Ackman, R.G. Capillary Column Gas Chromatographic Method for Analysis of Encapsulated Fish Oils and Fish Oil Ethyl Esters: Collaborative Study. J. AOAC Int. 1992, 75, 488–506. [Google Scholar] [CrossRef]
- Cho, C.Y. Fish nutrition, feeds, and feeding: With special emphasis on salmonid aquaculture. Food Rev. Int. 1990, 6, 333–357. [Google Scholar] [CrossRef]
- Bureau, D.; Harris, A.; Cho, C. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Apparent protein and energy digestibility of common and alternative feed ingredients by Atlantic cod, Gadus morhua (Linnaeus, 1758). Aquaculture 2006, 261, 1314–1327. [Google Scholar] [CrossRef]
- Furukawa, A.; Tsukahara, H. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull. Jpn. Soc. Sci. Fish. 1966, 32, 502–508. [Google Scholar] [CrossRef]
- Bureau, D.; Hua, K. Letter to the Editor of Aquaculture. Aquaculture 2006, 2, 103–105. [Google Scholar] [CrossRef]
- Panicz, R.; Eljasik, P.; Troszok, A.; Sobczak, M.; Lisiecki, S.; Nędzarek, A.; Sadowski, J. Safe management of Cyprinid herpesvirus 3-induced mortalities of common carp (Cyprinus carpio) by silaging process. Aquac. Rep. 2022, 24, 101116. [Google Scholar] [CrossRef]
- Arason, S. Production of fish silage. In Fisheries Processing; Springer: Boston, MA, USA, 1994; pp. 244–272. [Google Scholar]
- Millamena, O.M. Replacement of fish meal by animal by-product meals in a practical diet for grow-out culture of grouper Epinephelus coioides. Aquaculture 2002, 204, 75–84. [Google Scholar] [CrossRef]
- Ozyurt, G.; Gokdogan, S.; Şimşek, A.; Yuvka, I.; Ergüven, M.; Kuley Boga, E. Fatty acid composition and biogenic amines in acidified and fermented fish silage: A comparison study. Arch. Anim. Nutr. 2016, 70, 72–86. [Google Scholar] [CrossRef]
- Zahar, M.; Benkerroum, N.; Guerouali, A.; Laraki, Y.; El Yakoubi, K. Effect of temperature, anaerobiosis, stirring and salt addition on natural fermentation silage of sardine and sardine wastes in sugarcane molasses. Bioresour. Technol. 2002, 82, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Pleje, M. Silage fermentation of fish or fish waste products with lactic acid bacteria. J. Sci. Food Agric. 1983, 34, 1057–1067. [Google Scholar] [CrossRef]
- Land, M.V.; Vanderperren, E.; Raes, K. The effect of raw material combination on the nutritional composition and stability of four types of autolyzed fish silage. Anim. Feed. Sci. Technol. 2017, 234, 284–294. [Google Scholar] [CrossRef]
- Zieliński, M.; Rusanowska, P.; Zielińska, M.; Dudek, M.; Nowicka, A.; Purwin, C.; Fijałkowska, M.; Dębowski, M. Influence of preparation of Sida hermaphrodita silages on its conversion to methane. Renew. Energy 2021, 163, 437–444. [Google Scholar] [CrossRef]
- Guimarães, I.G.; Martins, G.P. Nutritional requirement of two Amazonian aquacultured fish species, Colossoma macropomum (Cuvier, 1816) and Piaractus brachypomus (Cuvier, 1818): A mini review. J. Appl. Ichthyol. 2015, 31, 57–66. [Google Scholar] [CrossRef]
- Shabani, A.; Boldaji, F.; Dastar, B.; Ghoorchi, T.; Zerehdaran, S.; Ashayerizadeh, A. Evaluation of increasing concentrations of fish waste silage in diets on growth performance, gastrointestinal microbial population, and intestinal morphology of broiler chickens. Anim. Feed. Sci. Technol. 2021, 275, 114874. [Google Scholar] [CrossRef]
- Miao, S.; Chang, E.; Han, B.; Zhang, X.; Liu, X.; Zhou, Z.; Zhou, Y. Dietary tryptophan requirement of northern snakehead, Channa argus (Cantor, 1842). Aquaculture 2021, 542, 736904. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef]
- dos Santos, D.K.M.; Santana, T.M.; Dantas, F.D.M.; Farias, A.B.d.S.; Epifânio, C.M.F.; Prestes, A.G.; da Fonseca, F.A.L.; Parisi, G.; Viegas, E.M.M.; Gonçalves, L.U. Defatted black soldier fly larvae meal as a dietary ingredient for tambaqui (Colossoma macropomum): Digestibility, growth performance, haematological parameters, and carcass composition. Aquac. Res. 2022, 53, 6762–6770. [Google Scholar] [CrossRef]
- Goosen, N.J.; de Wet, L.F.; Görgens, J.F. Rainbow trout silage as immune stimulant and feed ingredient in diets for Mozambique tilapia (Oreochromis mossambicus). Aquac. Res. 2016, 47, 329–340. [Google Scholar] [CrossRef]
- Islam, J.; Peñarubia, O. Seafood Waste Management Status in Bangladesh and Potential for Silage Production. Sustainability 2021, 13, 2372. [Google Scholar] [CrossRef]


| Ingredient (%) | Reference Diet | Molasses VS | Wheat VS | Cassava VS | Acid VS |
|---|---|---|---|---|---|
| Soybean meal | 47.3 | 33.04 | 33.04 | 33.04 | 33.04 |
| Corn meal | 39.2 | 27.38 | 27.38 | 27.38 | 27.38 |
| Wheat meal | 6.3 | 4.40 | 4.40 | 4.40 | 4.40 |
| Meat and bone meal | 5.0 | 3.49 | 3.49 | 3.49 | 3.49 |
| Molasses VS | - | 30.0 | - | - | - |
| Wheat VS | - | - | 30.0 | - | - |
| Cassava VS | - | - | - | 30.0 | - |
| Acid VS | - | - | - | - | 30.0 |
| Vitamin/mineral supplement a | 1.0 | 0.70 | 0.70 | 0.70 | 0.70 |
| Dicalcium phosphate | 0.5 | 0.35 | 0.35 | 0.35 | 0.35 |
| Salt | 0.1 | 0.07 | 0.07 | 0.07 | 0.07 |
| DL-Methionine | 0.08 | 0.06 | 0.06 | 0.06 | 0.06 |
| BHT | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Chromium oxide III | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Proximate composition (%) | |||||
| Dry matter | 96.10 | 96.63 | 96.97 | 96.83 | 96.97 |
| Crude protein | 31.90 | 28.43 | 29.07 | 28.90 | 29.10 |
| Crude lipid | 3.40 | 16.05 | 14.15 | 14.35 | 19.20 |
| Crude fiber | 4.16 | 3.72 | 4.54 | 3.61 | 7.75 |
| Ash | 8.05 | 7.05 | 7.00 | 6.95 | 6.95 |
| Calcium | 0.54 | 0.60 | 0.68 | 0.65 | 0.63 |
| Phosphor | 0.42 | 0.39 | 0.38 | 0.38 | 0.39 |
| Gross Energy (kcal·100 g−1) b | 310.23 | 331.19 | 305.00 | 327.40 | 334.63 |
| Composition | Fish Viscera | Molasses VS | Wheat VS | Cassava VS | Acid VS | p-Value |
|---|---|---|---|---|---|---|
| Dry matter | 52.4 ± 1.7 | 45.8 ± 0.4 c | 62.4 ± 0.9 a | 56.1 ± 0.6 b | 55.7 ± 0.2 b | <0.001 |
| Crude protein | 30.8 ± 0.4 | 13.1 ± 1.0 a | 11.9 ± 0.5 ab | 9.8 ± 1.5 b | 13.3 ± 0.5 a | <0.001 |
| Crude lipid | 29.4 ± 0.6 | 59.3 ± 0.6 b | 60.5 ± 0.6 b | 61.9 ± 1.1 ab | 69.9 ± 0.2 a | <0.001 |
| Crude fiber | 1.4 ± 0.2 | 1.2 ± 0.1 b | 5.41 ± 0.7 ab | 9.5 ± 0.4 a | 0.1 ± 0.02 c | <0.001 |
| Ash | 18.9 ± 0.5 | 5.3 ± 0.9 ab | 1.7 ± 0.2 b | 4.4 ± 0.6 ab | 10.3 ± 0.5 a | <0.001 |
| Gross energy (kcal·100 g−1) i | 405.7 ± 4.3 | 608.5 ± 53.3 b | 629.4 ± 105.1 ab | 611.1 ± 121.0 b | 686.6 ± 28.5 a | <0.001 |
| Amino Acids | Fish Viscera | Molasses VS | Wheat VS | Cassava VS | Acid VS | p-Value |
|---|---|---|---|---|---|---|
| Essential | ||||||
| Arginine | 9.4 ± 0.3 | 2.0 ± 0.0 b | 3.3 ± 0.1 b | 2.0 ± 0.1 b | 8.9 ± 0.1 a | <0.001 |
| Histidine | 2.9 ± 0.16 | 1.9 ± 0.0 b | 2.3 ± 0.0 b | 1.9 ± 0.0 b | 3.1 ± 0.0 a | <0.001 |
| Isoleucine | 5.3 ± 0.1 | 3.4 ± 0.0 b | 3.7 ± 0.0 b | 3.8 ± 0.0 b | 5.0 ± 0.1 a | 0.002 |
| Leucine | 10.5 ± 0.2 | 5.6 ± 0.1 b | 7.4 ± 0.0 b | 7.0 ± 0.1 b | 10.2 ± 0.1 a | <0.001 |
| Lysine | 8.7 ± 0.1 | 5.2 ± 0.0 b | 3.7 ± 0.0 c | 3.6 ± 0.1 c | 8.9 ± 0.1 a | <0.001 |
| Methionine | 3.4 ± 0.1 | 1.8 ± 0.0 b | 1.9 ± 0.0 b | 1.9 ± 0.0 b | 3.2 ± 0.0 a | <0.001 |
| Phenylalanine | 5.3 ± 0.2 | 2.7 ± 0.0 c | 3.8 ± 0.0 b | 3.4 ± 0.0 bc | 5.4 ± 0.1 a | <0.001 |
| Threonine | 5.8 ± 0.2 | 3.2 ± 0.0 c | 4.2 ± 0.0 b | 3.8 ± 0.0 bc | 5.7 ± 0.1 a | <0.001 |
| Tryptophan | 1.2 ± 0.1 | 1.0 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.1 | 1.3 ± 0.0 | ns |
| Valine | 6.7 ± 0.2 | 4.3 ± 0.0 b | 5.1 ± 0.0 b | 4.8 ± 0.1 b | 6.7 ± 0.1 a | 0.001 |
| Nonessential | ||||||
| Aspartic acid | 11.3 ± 0.2 | 4.2 ± 0.0 c | 7.3 ± 0.1 b | 5.6 ± 0.1 bc | 10.9 ± 0.1 a | <0.001 |
| Alanine | 16.4 ± 0.1 | 7.6 ± 0.0 | 8.7 ± 0.1 | 8.1 ± 0.1 | 9.7 ± 0.1 | ns |
| Cystine | 10.2 ± 0.4 | 0.0 ± 0.0 b | 0.2 ± 0.0 b | 0.0 ± 0.0 b | 1.2 ± 0.0 a | <0.001 |
| Glycine | 0.9 ± 0.1 | 6.0 ± 0.0 b | 11.3 ± 0.2 a | 9.3 ± 0.3 ab | 13.6 ± 0.1 a | 0.006 |
| Glutamic acid | 14.2 ± 0.7 | 9.1 ± 0.0 c | 13.6 ± 0.1 a | 10.4 ± 0.1 bc | 15.3 ± 0.2 a | 0.001 |
| Serine | 5.4 ± 0.2 | 3.1 ± 0.0 bc | 3.8 ± 0.0 b | 2.9 ± 0.0 c | 5.3 ± 0.1 a | <0.001 |
| Proline | 9.2 ± 0.4 | 4.4 ± 0.0 b | 7.8 ± 0.1 a | 6.3 ± 0.1 ab | 8.7 ± 0.1 a | 0.004 |
| Taurine | 1.7 ± 0.0 | 1.4 ± 0.0 b | 1.2 ± 0.0 b | 1.3 ± 0.0 b | 2.2 ± 0.0 a | <0.001 |
| Tyrosine | 4.1 ± 0.2 | 1.0 ± 0.0 b | 0.8 ± 0.0 b | 0.9 ± 0.0 b | 4.0 ± 0.0 a | <0.001 |
| Crude protein (%) | 30.8 | 13.1 | 11.9 | 9.8 | 13.3 |
| Fatty Acids | Fish Viscera | Molasses VS | Wheat VS | Cassava VS | Acid VS | p-Value |
|---|---|---|---|---|---|---|
| Lauric (12:0) | nd | 5.2 ± 0.3 | 4.0 ± 2.6 | 2.7 ± 1.8 | 6.5 ± 1.9 | ns |
| Myristic (14:0) | 13.7 ± 0.0 | 15.8 ± 2.2 | 12.9 ± 1.9 | 14.7 ± 1.3 | 15.2 ± 1.8 | ns |
| Palmitic (16:0) | 309.6 ± 0.1 | 239.4 ± 6.0 | 254.8 ± 0.5 | 257.3 ± 28.1 | 271.7 ± 15.8 | ns |
| Heptadecanoic acid (17:0) | 1.2 ± 0.0 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.2 | 1.6 ± 0.2 | ns |
| Stearic acid (18:0) | 162.8 ± 0.1 | 124.0 ± 4.8 | 140.6 ± 17.4 | 121.6 ± 11.5 | 132.3 ± 4.3 | ns |
| Arachidic acid (20:0) | 3.1 ± 0.0 | 4.8 ± 1.4 | 4.4 ± 0.7 | 4.6 ± 0.5 | 4.8 ± 0.6 | ns |
| Heneicosanoic acid (21:0) | 3.2 ± 0.0 | 6.1 ± 0.2 | 3.6 ± 0.5 | 4.2 ± 0.4 | 4.3 ± 0.5 | ns |
| ∑SFAs | 493.6 ± 0.2 | 396.9 ± 1.6 | 421.9 ± 15.1 | 406.6 ± 34.8 | 436.4 ± 18.3 | ns |
| Palmitoleic acid (16:1n-7) | 27.3 ± 0.0 | 33.9 ± 3.3 | 32.5 ± 4.3 | 36.4 ± 1.3 | 3.15 ± 0.1 | ns |
| Palmitoleic acid (16:1n-9) | 3.5 ± 0.0 | 5.0 ± 0.7 | 7.7 ± 2.7 | 6.1 ± 1.5 | 0.48 ± 0.0 | ns |
| Vaccenic acid (18:1n-7) | 16.4 ± 0.0 | 23.1 ± 4.6 | 19.3 ± 0.8 | 21.5 ± 1.3 | 1.76 ± 0.2 | ns |
| Oleic acid (18:1n-9) | 380.5 ± 0.0 | 434.7 ± 2.9 | 406.7 ± 24.4 | 425.2 ± 37.0 | 403.9 ± 2.9 | ns |
| ∑MUFAs | 427.7 ± 0.1 | 496.7 ± 2.0 | 466.2 ± 17.5 | 489.2 ± 17.0 | 409.3 ± 2.93 | ns |
| Linoleic acid (18:2n-6) | 58.4 ± 0.1 | 72.9 ± 1.6 | 70.6 ± 3.9 | 72.4 ± 2.5 | 67.6 ± 2.9 | ns |
| Linolenic acid (18:3n-3) | 15.5 ± 0.0 | 17.5 ± 2.9 | 19.6 ± 5.6 | 16.7 ± 4.5 | 18.9 ± 7.6 | ns |
| Arachidonic acid (20:4n-6) | 3.0 ± 0.0 | 5.2 ± 1.1 | 4.1 ± 0.8 | 6.2 ± 3.1 | 4.2 ± 0.8 | ns |
| Eicosapentaenoic acid (20:5n-3) | 1.1 ± 0.1 | 5.4 ± 6.4 | 17.8 ± 15.8 | 8.2 ± 1.4 | 10.0 ± 1.28 | ns |
| Docosahexaenoic acid (22:6 n-3) | 0.5 ± 0.0 | 1.7 ± 2.5 | 6.7 ± 5.5 | 1.7 ± 2.0 | 8.9 ± 1.18 | ns |
| ∑PUFAs | 78.3 ± 0.22 | 102. 7 ± 3.8 | 118.8 ± 23.2 | 105.2 ± 3.8 | 109.6 ± 15.6 | ns |
| ∑n-6 | 61.4 ± 0.08 | 78.1 ± 1.3 | 74.7 ± 4.5 | 78.6 ± 3.9 | 71.8 ± 3.6 | ns |
| ∑n-3 | 17.1 ± 0.08 | 24.6 ± 3.3 | 55.9 ± 11.3 | 27.3 ± 2.5 | 46.7 ± 6.5 | ns |
| n-6:n-3 | 3.6 ± 0.4 | 3.2 ± 0.4 | 1.4 ± 0.4 | 2.9 ± 0.4 | 1.6 ± 0.3 | ns |
| Composition | Molasses VS | Wheat VS | Cassava VS | Acid VS | p-Value |
|---|---|---|---|---|---|
| Dry matter | 79.46 ± 2.7 | 86.88 ± 6.6 | 78.77 ± 3.2 | 82.97 ± 5.6 | ns |
| Crude protein | 88.32 ± 0.6 ab | 91.94 ± 2.9 a | 86.91 ± 0.3 ab | 84.24 ± 4.4 b | 0.040 |
| Crude lipid | 94.96 ± 1.8 | 94.60 ± 1.2 | 94.10 ± 0.8 | 93.57 ± 0.1 | ns |
| Gross energy | 85.06 ± 2.8 | 84.20 ± 8.7 | 82.64 ± 1.2 | 87.15 ± 6.9 | ns |
| Calcium | 76.45 ± 2.0 b | 90.98 ± 1.6 a | 90.58 ± 7.2 a | 89.11 ± 4.6 a | 0.039 |
| Phosphorus | 81.20 ± 11.4 | 80.69 ± 8.8 | 76.95 ± 11.5 | 80.09 ± 4.9 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, T.M.; Dantas, F.d.M.; Monteiro Dos Santos, D.K.; Kojima, J.T.; Pastrana, Y.M.; De Jesus, R.S.; Gonçalves, L.U. Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles. Fishes 2023, 8, 111. https://doi.org/10.3390/fishes8020111
Santana TM, Dantas FdM, Monteiro Dos Santos DK, Kojima JT, Pastrana YM, De Jesus RS, Gonçalves LU. Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles. Fishes. 2023; 8(2):111. https://doi.org/10.3390/fishes8020111
Chicago/Turabian StyleSantana, Thiago Macedo, Francisco de Matos Dantas, Driely Kathriny Monteiro Dos Santos, Juliana Tomomi Kojima, Yugo Moraes Pastrana, Rogério Souza De Jesus, and Ligia Uribe Gonçalves. 2023. "Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles" Fishes 8, no. 2: 111. https://doi.org/10.3390/fishes8020111
APA StyleSantana, T. M., Dantas, F. d. M., Monteiro Dos Santos, D. K., Kojima, J. T., Pastrana, Y. M., De Jesus, R. S., & Gonçalves, L. U. (2023). Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles. Fishes, 8(2), 111. https://doi.org/10.3390/fishes8020111