Profiling a New Postbiotic Product for Its Application in Fish Aquaculture
Abstract
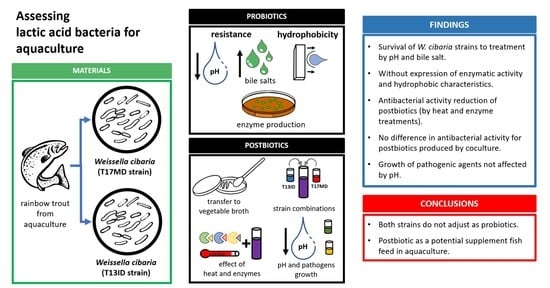
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Pathogens
2.3. Resistance to Bile Salts and pH Values
2.4. Hydrophobicity Test
2.5. Enzyme Production
2.6. Postbiotic Production
2.7. Transfer to New Culture Media
2.8. Strain Combinations
2.9. Effect of Heat and Enzymes on Antibacterial Activity
2.10. Influence of pH on Pathogen Growth
2.11. Statistical Analysis
3. Results
3.1. Resistance to Bile Salts, pH, and Hydrophobicity Test
3.2. Enzyme Production and Biochemical Analysis
3.3. Transfer to the New Culture Median and Coculture of Strains
3.4. Sensitivity of the Postbiotic Product
3.5. Influence of Acidic pH on Pathogen Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, C.G.; Fusieger, A.; Milião, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An Emerging Bacterium with Promising Health Benefits. Probiotics Antimicrob. 2021, 13, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Montemurro, M.; Chieffi, D.; Cho, G.S.; Franz CM, A.P.; Dell’Aquila, A.; Rizzello, C.G.; Fusco, V. Novel Insights into the Phylogeny and Biotechnological Potential of Weissella Species. Front. Microbiol. 2022, 13, 914036. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.R.; Kothari, D.; Shukla, R.; Goyal, A. Structural and biocompatibility properties of dextran from Weissella cibaria JAG8 as food additive. Int. J. Food Sci. Nutr. 2014, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-forming Weissella strains as starter cultures for sorghum and wheat sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Srionnual, S.; Yanagida, F.; Lin, L.H.; Hsiao, K.N.; Chen, Y.S. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl. Environ. Microbiol. 2007, 73, 2247–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Benno, Y.; Nakase, T.; Oh, T.-K. Specific probiotic characterization of Weissella hellenica DS-12 isolated from flounder intestine. J. Gen. Appl. Microbiol. 1998, 44, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.-K.; Yang, B.-T.; Hao, Z.-P.; Li, H.-Z.; Cong, W.; Kang, Y.-H. Dietary supplementation with Weissella cibaria C-10 and Bacillus amyloliquefaciens T-5 enhance immunity against Aeromonas veronii infection in crucian carp (Carassiu auratus). Microb. Pathog. 2022, 167, 105559. [Google Scholar] [CrossRef]
- Quintanilla-Pineda, M.; Garrote Achou, C.; Díaz, J.; Gutiérrez-Falcon, A.; Bravo, M.; Herrera-Muñoz, J.I.; Peña-Navarro, N.; Alvarado, C.; Ibañez, F.C.; Marzo, F. In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida. Foods 2023, 12, 861. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations and World Health Organization. Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Food and Agricultural Organization of the United Nations and World Health Organization: Córdoba, Argentina, 2001; Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 5 May 2023).
- Lee, K.W.; Park, J.Y.; Jeong, H.R.; Heo, H.J.; Han, N.S.; Kim, J.H. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 2012, 18, 96–102. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Abriouel, H.; Lerma, L.L.; Muñoz, M.D.C.C.; Montoro, B.P.; Kabisch, J.; Pichner, R.; Cho, G.S.; Neve, H.; Fusco, V.; Franz, C.M.A.P.; et al. The controversial nature of the Weissella genus: Technological and functional aspects versus whole genome analysis-based pathogenic potential for their application in food and health. Front. Microbiol. 2015, 6, 1197. [Google Scholar] [CrossRef] [Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Medina, M.; Sotil, G.; Flores, V.; Fernández, C.; Sandoval, N. In vitro assessment of some probiotic properties and inhibitory activity against Yersinia ruckeri of bacteria isolated from rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Rep. 2020, 18, 100447. [Google Scholar] [CrossRef]
- Xu, H.; Jeong, H.S.; Lee, H.Y.; Ahn, J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett. Appl. Microbiol. 2009, 49, 434–442. [Google Scholar] [CrossRef]
- Jiménez Paillié, E.M. Determinación de la Actividad Celulolítica, Ligninolítica y Amilolítica de Actinobacterias Aisladas de Suelo Rizosférico de Trébol Blanco (Trifolium repens). Undergraduate Thesis, Pontificia Universidad Javeriana, Bogotá D.C., Colombia, 2012. [Google Scholar]
- Touraki, M.; Frydas, I.; Karamanlidou, G.; Mamara, A. Partial purification and characterization of a bacteriocin produced by Bacillus subtilis NCIMB 3610 that exhibits antimicrobial activity against fish pathogens. J. Biol. Res. 2012, 18, 310–319. [Google Scholar]
- Ang, C.Y.; Sano, M.; Dan, S.; Leelakriangsak, M.; Lal, T.M. Postbiotics Applications as Infectious Disease Control Agent in Aquaculture. Biocontrol Sci. 2020, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Teame, T.; Hao, Q.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Zhang, Y.; Zhou, Z.; Duan, M.; et al. Use of a paraprobiotic and postbiotic feed supplement (HWFTM) improves the growth performance, composition and function of gut microbiota in hybrid sturgeon (Acipenser baerii x Acipenser schrenckii). Fish Shellfish Immunol. 2020, 104, 36–45. [Google Scholar] [CrossRef]
- Dash, G.; Raman, R.P.; Pani Prasad, K.; Makesh, M.; Pradeep, M.A.; Sen, S. Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Fish Shellfish Immunol. 2015, 43, 167–174. [Google Scholar] [CrossRef]
- Mortezaei, F.; Royan, M.; Allaf Noveirian, H.; Babakhani, A.; Alaie Kordghashlaghi, H.; Balcázar, J.L. In vitro assessment of potential probiotic characteristics of indigenous Lactococcus lactis and Weissella oryzae isolates from rainbow trout (Oncorhynchus mykiss Walbaum). J. Appl. Microbiol. 2020, 129, 1004–1019. [Google Scholar] [CrossRef]
- Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Schusterová, P.; Cingeľová Maruščáková, I.; Popelka, P. Characterization of two novel lactic acid bacteria isolated from the intestine of rainbow trout (Oncorhynchus mykiss, Walbaum) in Slovakia. Aquaculture 2019, 506, 294–301. [Google Scholar] [CrossRef]
- Sica, M.G.; Brugnoni, L.I.; Marucci, P.L.; Cubitto, M.A. Characterization of probiotic properties of lactic acid bacteria isolated from an estuarine environment for application in rainbow trout (Oncorhynchus mykiss, Walbaum) farming. Antonie van Leeuwenhoek 2012, 101, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pundir, R.K. Effect of fermentation medium, pH and temperature variations on antibacterial soil fungal metabolite production. J. Agric. Technol. 2011, 7, 247–269. [Google Scholar]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Rühs, P.A.; Böcker, L.; Inglis, R.F.; Fischer, P. Studying bacterial hydrophobicity and biofilm formation at liquid-liquid interfaces through interfacial rheology and pendant drop tensiometry. Colloids Surf. B 2014, 117, 174–184. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Bravo Santillana, M. Caracterización de Bacterias Ácido-Lácticas con Propiedades Antimicrobianas e Inmunomoduladoras y su Investigación Aplicada en Sanidad. Ph.D. Thesis, Universidad de Extremadura, Extremadura, Spain, 2021. [Google Scholar]
- Mirkovic, N.; Radulovic, Z.; Uzelac, G.; Lozo, J.; Obradovic, D.; Topisirovic, L.; Kojic, M. Isolation and Characterisation of Bacteriocin and Aggregation-Promoting Factor Production in Lactococcus lactis ssp. lactis BGBM50 Strain. Food Technol. Biotechnol. 2015, 53, 237–242. [Google Scholar] [CrossRef]
- Bravo, M.; Combes, T.; Martinez, F.O.; Cerrato, R.; Rey, J.; Garcia-Jimenez, W.; Fernandez-Llario, P.; Risco, D.; Gutierrez-Merino, J. Lactobacilli Isolated from Wild Boar (Sus scrofa) Antagonize Mycobacterium bovis Bacille Calmette-Guerin (BCG) in a Species-Dependent Manner. Front. Microbiol. 2019, 10, 1663. [Google Scholar] [CrossRef] [Green Version]

| Counts (Log CFU/mL) | ||
|---|---|---|
| Treatment | T13ID | T17MD |
| Bile 10% | 8.97 ± 0.04 b | 9.24 ± 0.05 a |
| Bile 20% | 8.70 ± 0.00 c | 9.21 ± 0.00 a |
| pH 3 | 9.30 ± 0.00 a | 9.22 ± 0.02 a |
| pH 4 | 9.39 ± 0.08 a | 9.16 ± 0.04 b |
| pH 5 | 9.05 ± 0.02 b | 9.19 ± 0.04 a |
| Control | 9.06 ± 0.02 b | 9.30 ± 0.02 a |
| Strain | 24 h | 48 h | 72 h | Standard | Vegetal | |||
|---|---|---|---|---|---|---|---|---|
| Counts | pH | Counts | pH | Counts | pH | pH | pH | |
| T17MD | ||||||||
| Vegetal broth | 9.18 ± 0.07 b | 4.12 ± 0.01 a | 8.60 ± 0.04 d | 4.03 ± 0.01 a | 3.83 ± 0.10 a | 4.02 ± 0.01 a | 6.40 ± 0.00 | 6.90 ± 0.00 |
| Standard broth | 9.42 ± 0.02 a | 4.44 ± 0.01 b | 9.24 ± 0.02 a | 4.43 ± 0.00 c | 0.00 ± 0.00 b | 4.49 ± 0.00 b | 6.40 ± 0.00 | 6.90 ± 0.00 |
| T13ID | ||||||||
| Vegetal broth | 9.06 ± 0.07 b | 4.12 ± 0.02 a | 8.53 ± 0.02 e | 4.02 ± 0.01 a | 0.00 ± 0.00 b | 4.02 ± 0.00 a | 6.40 ± 0.00 | 6.90 ± 0.00 |
| Standard broth | 9.32 ± 0.01 a | 4.42 ± 0.01 b | 9.07 ± 0.02 b | 4.42 ± 0.00 c | 0.00 ± 0.00 Bb | 4.47 ± 0.06 b | 6.40 ± 0.00 | 6.90 ± 0.00 |
| T17MD + T13ID | ||||||||
| Vegetal broth | 9.30 ± 0.03 a | 4.10 ± 0.02 a | 6.23 ± 0.05 f | 4.09 ± 0.00 b | 0.00 ± 0.00 b | 4.02 ± 0.02 a | 6.40 ± 0.00 | 6.90 ± 0.00 |
| Standard broth | 9.14 ± 0.08 b | 4.48 ± 0.00 c | 8.86 ± 0.03 c | 4.48 ± 0.01 d | 0.00 ± 0.00 b | 4.48 ± 0.01 b | 6.40 ± 0.00 | 6.90 ± 0.00 |
| Strains | A. salmonicida subsp. salmonicida | Y. ruckeri |
|---|---|---|
| T17MD | ||
| Vegetal broth | 1.47 ± 0.21 c | 0.24 ± 0.07 b |
| Standard broth | 4.24 ± 0.05 b | 0.30 ± 0.12 b |
| T13ID | ||
| Vegetal broth | 1.21 ± 0.03 c | 0.20 ± 0.02 b |
| Standard broth | 4.64 ± 0.26 a | 0.26 ± 0.05 b |
| T17MD + T13ID | ||
| Vegetal broth | 1.40 ± 0.10 c | 0.22 ± 0.07 b |
| Standard broth | 3.46 ± 0.10 b | 0.40 ± 0.04 a |
| Control | 0.00 ± 0.00 d | 0.00 ± 0.00 c |
| Treatment | A. Salmonicida subsp. salmonicida | Y. ruckeri |
|---|---|---|
| 95 °C/12 s | 3.30 ± 0.05 b | 0.57 ± 0.05 a |
| 95 °C/30 s | 3.28 ± 0.04 b | 0.31 ± 0.08 bcd |
| 105 °C/12 s | 3.55 ± 0.16 ab | 0.27 ± 0.01 cd |
| 105 °C/30 s | 3.49 ± 0.07 ab | 0.34 ± 0.03 bc |
| 130 °C/12 s | 2.53 ± 0.17 c | 0.39 ± 0.04 b |
| 130 °C/30 s | 2.29 ± 0.10 c | 0.23 ± 0.05 d |
| Control (+) | 3.70 ± 0.26 a | 0.34 ± 0.06 bc |
| Control (−) | 0.00 ± 0.00 d | 0.00 ± 0.00 e |
| pH | Y. ruckeri | A. salmonicida subsp. salmonicida |
|---|---|---|
| 3 | 8.92 ± 0.05 | 8.77 ± 0.09 |
| 4 | 8.88 ± 0.01 | 8.68 ± 0.15 |
| 5 | 8.90 ± 0.07 | 8.65 ± 0.07 |
| Control | 8.92 ± 0.06 | 8.82 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Pineda, M.; Díaz, J.; Gutiérrez-Falcon, A.; Ibañez, F.C.; Marzo, F. Profiling a New Postbiotic Product for Its Application in Fish Aquaculture. Fishes 2023, 8, 304. https://doi.org/10.3390/fishes8060304
Quintanilla-Pineda M, Díaz J, Gutiérrez-Falcon A, Ibañez FC, Marzo F. Profiling a New Postbiotic Product for Its Application in Fish Aquaculture. Fishes. 2023; 8(6):304. https://doi.org/10.3390/fishes8060304
Chicago/Turabian StyleQuintanilla-Pineda, Mario, Jesús Díaz, Ana Gutiérrez-Falcon, Francisco C. Ibañez, and Florencio Marzo. 2023. "Profiling a New Postbiotic Product for Its Application in Fish Aquaculture" Fishes 8, no. 6: 304. https://doi.org/10.3390/fishes8060304
APA StyleQuintanilla-Pineda, M., Díaz, J., Gutiérrez-Falcon, A., Ibañez, F. C., & Marzo, F. (2023). Profiling a New Postbiotic Product for Its Application in Fish Aquaculture. Fishes, 8(6), 304. https://doi.org/10.3390/fishes8060304