Transcriptomic Analysis Reveals Circadian Rhythm Homeostasis in Pearl Gentian Grouper under Acute Hypoxia
Abstract
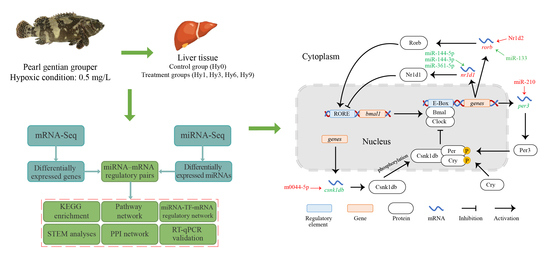
:1. Introduction
2. Materials and Methods
2.1. Acute Hypoxic Stress and Sampling
2.2. MiRNA Sequencing and Prediction of miRNA Target Genes
2.3. KEGG Enrichment, Pathway Network, and STEM Analyses
2.4. Construction of a PPI Network and miRNA-TF-mRNA Regulatory Network Based on Target Genes
2.5. RT-qPCR Validation Experiment
3. Results
3.1. KEGG Enrichment and Pathway Network Analyses
3.2. Expression Trends of Target Genes
3.3. PPI Network and miRNA-TF-mRNA Regulatory Network of Circadian Rhythm Genes
3.4. RT-qPCR Validation of Key Genes and miRNAs
4. Discussion
4.1. Expression Changes of Core Clock Genes and Stability of the Circadian Cycle under Hypoxia
4.2. Regulation of the Auxiliary Loop and Stability of Circadian Clock Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunlap, J.C. Molecular bases for circadian clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmer, S.L.; Panda, S.; Kay, S.A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001, 17, 215–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philpott, J.M.; Torgrimson, M.R.; Harold, R.L.; Partch, C.L. Biochemical mechanisms of period control within the mammalian circadian clock. Semin. Cell Dev. Biol. 2022, 126, 71–78. [Google Scholar] [CrossRef]
- Morris, A.R.; Stanton, D.L.; Roman, D.; Liu, A.C. Systems level understanding of circadian integration with cell physiology. J. Mol. Biol. 2020, 432, 3547–3564. [Google Scholar] [CrossRef]
- Rey, G.; Reddy, A.B. Connecting cellular metabolism to circadian clocks. Trends Cell Biol. 2013, 23, 234–241. [Google Scholar] [CrossRef]
- Amaral, I.P.G.; Johnston, I.A. Circadian expression of clock and putative clock-controlled genes in skeletal muscle of the zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R193–R206. [Google Scholar] [CrossRef]
- Solt, L.A.; Kojetin, D.J.; Burris, T.P. The REV-ERBs and RORs: Molecular links between circadian rhythms and lipid homeostasis. Future Med. Chem. 2011, 3, 623–638. [Google Scholar] [CrossRef] [Green Version]
- Covington, M.; Maloof, J.; Straume, M.; Kay, S.; Harmer, S. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef] [Green Version]
- Krittika, S.; Yadav, P. Circadian clocks: An overview on its adaptive significance. Biol. Rhythm Res. 2020, 51, 1109–1132. [Google Scholar] [CrossRef]
- Reebs, S. Plasticity of diel and circadian rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- Costa, L.S.; Serrano, I.; Sánchez-Vázquez, F.J.; López-Olmeda, J.F. Circadian rhythms of clock gene expression in nile tilapia (Oreochromis niloticus) central and peripheral tissues: Influence of different lighting and feeding conditions. J. Comp. Physiol. B 2016, 186, 775–785. [Google Scholar] [CrossRef]
- Prokkola, J.M.; Nikinmaa, M. Circadian rhythms and environmental disturbances—Underexplored interactions. J. Exp. Biol. 2018, 221, jeb179267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Olmeda, J.F.; Madrid, J.A.; Sánchez-Vázquez, F.J. Light and temperature cycles as zeitgebers of zebrafish (Danio rerio) circadian activity rhythms. Chronobiol. Int. 2006, 23, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Mortola, J.P. Hypoxia and circadian patterns. Respir. Physiol. Neurobiol. 2007, 158, 274–279. [Google Scholar] [CrossRef]
- Sandbichler, A.M.; Jansen, B.; Peer, B.A.; Paulitsch, M.; Pelster, B.; Egg, M. Metabolic plasticity enables circadian adaptation to acute hypoxia in zebrafish cells. Cell. Physiol. Biochem. 2018, 46, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Egg, M.; Köblitz, L.; Hirayama, J.; Schwerte, T.; Folterbauer, C.; Kurz, A.; Fiechtner, B.; Möst, M.; Salvenmoser, W.; Sassone-Corsi, P.; et al. Linking oxygen to time: The bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol. Int. 2013, 30, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Prokkola, J.M.; Nikinmaa, M.; Lubiana, P.; Kanerva, M.; McCairns, R.J.S.; Götting, M. Hypoxia and the pharmaceutical diclofenac influence the circadian responses of three-spined stickleback. Aquat. Toxicol. 2015, 158, 116–124. [Google Scholar] [CrossRef]
- Svendsen, J.C.; Genz, J.; Anderson, W.G.; Stol, J.A.; Watkinson, D.A.; Enders, E.C. Evidence of circadian rhythm, oxygen regulation capacity, metabolic repeatability and positive correlations between forced and spontaneous maximal metabolic rates in lake sturgeon Acipenser fulvescens. PLoS ONE 2014, 9, e94693. [Google Scholar] [CrossRef] [Green Version]
- Pelster, B.; Egg, M. Multiplicity of hypoxia-inducible transcription factors and their connection to the circadian clock in the zebrafish. Physiol. Biochem. Zool. 2015, 88, 146–157. [Google Scholar] [CrossRef]
- Williams, K.J.; Cassidy, A.A.; Verhille, C.E.; Lamarre, S.G.; MacCormack, T.J. Diel cycling hypoxia enhances hypoxia-tolerance in rainbow trout (Oncorhynchus mykiss): Evidence of physiological and metabolic plasticity. J. Exp. Biol. 2019, 222, jeb.206045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, Y.; Wang, Z.; Xu, K.; Xiao, X.; Mu, W. Comparison of effects in sustained and diel-cycling hypoxia on hypoxia tolerance, histology, physiology and expression of clock genes in high latitude fish Phoxinus lagowskii. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 260, 111020. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Bang, I.C.; Park, J.Y.; Sade, A.; Choi, J.; Lee, S.M. Effects of dietary protein and lipid levels on growth performance, feed utilization and body composition of juvenile hybrid grouper, Epinephelus fuscoguttatus × E. lanceolatus. Aquaculture 2015, 446, 283–289. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Ding, N.; Xiong, W.; Zheng, G.; Lin, Q.; Zhang, G. Effects of temperature on the survival, feeding, and growth of pearl gentian grouper (female Epinephelus fuscoguttatus × male Epinephelus lanceolatus). Fish. Sci. 2018, 84, 399–404. [Google Scholar] [CrossRef]
- Ding, S.X.; Liu, Q.H.; Wu, H.H.; Qu, M. A review of research advances on the biology and artificial breeding of groupers. J. Fish. Sci. China 2018, 25, 737–752. [Google Scholar] [CrossRef]
- Ruan, W.; Ji, W.W.; Zheng, L.; Yue, D.D.; Fang, H. On hypoxia stress in fish and its nutritional regulation and response. Mar. Fish. 2020, 42, 751–761. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wu, H.X.; Wu, L.; Ma, J.Z. Oxygen consumption rate and suffocation point of the juveniles for five species of mariculture fish. J. Mar. Sci. 2015, 33, 76–81. [Google Scholar] [CrossRef]
- Lin, G.W. The effect of water temperature, salinity and dissolved oxygen changes on survival for pearl gentian grouper (Epinephelus fuscoguttatus female × E. lanceolatus male). J. Aquac. 2020, 41, 29–32. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef]
- Liang, Y.S.; Wu, R.X.; Niu, S.F.; Miao, B.B.; Liang, Z.B.; Zhai, Y. Liver transcriptome analysis reveals changes in energy metabolism, oxidative stress, and apoptosis in pearl gentian grouper exposed to acute hypoxia. Aquaculture 2022, 561, 738635. [Google Scholar] [CrossRef]
- Lu, Z.F.; Huang, H.; Huang, X.M.; Huang, W.Z. Effects of hypoxic stress on antioxidant and energy metabolism of hybrid grouper (Epinephelus fuscoguttatus female × Epinephelus lanceolatus male). J. Guangdong Ocean. Univ. 2022, 42, 13–19. [Google Scholar] [CrossRef]
- Miao, B.B.; Niu, S.F.; Wu, R.X.; Liang, Z.B.; Zhai, Y. The microRNAs-transcription factors-mRNA regulatory network plays an important role in resistance to cold stress in the pearl gentian grouper. Front. Mar. Sci. 2022, 8, 824533. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Joachims, T. Support vector machines. In Learning to Classify Text Using Support Vector Madchines; Joachims, T., Ed.; Springer: Boston, MA, USA, 2002; pp. 35–44. [Google Scholar]
- Turner, D.A. Miranda: A non-strict functional language with polymorphic types. In Functional Programming Languages and Computer Architecture; Jouannaud, J.P., Ed.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–16. [Google Scholar]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Zheng, J.; Shen, N.; Wang, G.; Zhou, G.; Fang, Y.; Lin, J.; Zhao, J. Identification of pathways and genes associated with synovitis in osteoarthritis using bioinformatics analyses. Sci. Rep. 2018, 8, 10050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.M.; Chen, H.; Liu, W.; Liu, H.; Gong, J.; Wang, H.; Guo, A.Y. AnimalTFDB: A comprehensive animal transcription factor database. Nucleic Acids Res. 2012, 40, D144–D149. [Google Scholar] [CrossRef]
- Wang, H. Comparative analysis of period genes in teleost fish genomes. J. Mol. Evol. 2008, 67, 29–40. [Google Scholar] [CrossRef]
- Im, J.S.; Jung, B.H.; Kim, S.E.; Lee, K.H.; Lee, J.K. Per3, a circadian gene, is required for chk2 activation in human cells. FEBS Lett. 2010, 584, 4731–4734. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z.S.; Eichele, G.; Bradley, A.; et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Fahrenkrug, J.; Georg, B.; Hannibal, J.; Hindersson, P.; Gräs, S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 2006, 147, 3769–3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, I.C.; Hsiao, Y.C.; Sun, H.S.; Chen, T.M.; Lee, S.J. MicroRNAs regulate gene plasticity during cold shock in zebrafish larvae. BMC Genom. 2016, 17, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearman, L.P.; Jin, X.; Lee, C.; Reppert, S.M.; Weaver, D.R. Targeted disruption of the mPer3 gene: Subtle effects on circadian clock function. Mol. Cell. Biol. 2000, 20, 6269–6275. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M.; Weaver, D.R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001, 30, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Yagita, K.; Yamaguchi, S.; Tamanini, F.; van der Horst, G.T.; Hoeijmakers, J.H.; Yasui, A.; Loros, J.J.; Dunlap, J.C.; Okamura, H. Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev. 2000, 14, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Yúfera, M.; Perera, E.; Mata-Sotres, J.A.; Calduch-Giner, J.; Martínez-Rodríguez, G.; Pérez-Sánchez, J. The circadian transcriptome of marine fish (Sparus aurata) larvae reveals highly synchronized biological processes at the whole organism level. Sci. Rep. 2017, 7, 12943. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Shao, T.; Liao, X.; He, Y.; Wang, J.; Hu, P. Diurnal expression of circadian clock genes period 1 and period 3 in Pelteobagrus vachellii. J. Oceanol. Limnol. 2021, 39, 652–660. [Google Scholar] [CrossRef]
- Huang, X.; Le, Q.T.; Giaccia, A.J. MiR-210–Micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Nagel, R.; Clijsters, L.; Agami, R. The miRNA-192/194 cluster regulates the period gene family and the circadian clock. FEBS J. 2009, 276, 5447–5455. [Google Scholar] [CrossRef] [PubMed]
- Dilão, R.; Mota, B. The transcriptional regulation of PER protein in drosophila. J. Theor. Biol. 2019, 469, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, A.; Yáñez, J.M.; Foerster, C.; Aguirre, C.; Pereiro, L.; Burzio, V.; Moraga, M.; Reyes, A.E.; Antonelli, M. The CK1 gene family: Expression patterning in zebrafish development. Biol. Res. 2007, 40, 251–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Yuan, B.; Xie, P.; Gu, Y.; Liu, Z.; Wang, T.; Li, Z.; Xu, Y.; Liu, Y. Decoupling PER phosphorylation, stability and rhythmic expression from circadian clock function by abolishing PER-CK1 interaction. Nat. Commun. 2022, 13, 3991. [Google Scholar] [CrossRef]
- Lee, C.; Etchegaray, J.P.; Cagampang, F.R.A.; Loudon, A.S.I.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Lee, H.; Chen, R.; Lee, Y.; Yoo, S.; Lee, C. Essential roles of CKIδ and CKIε in the mammalian circadian clock. Proc. Natl. Acad. Sci. USA 2009, 106, 21359–21364. [Google Scholar] [CrossRef]
- Xu, P.; Ianes, C.; Gärtner, F.; Liu, C.; Burster, T.; Bakulev, V.; Rachidi, N.; Knippschild, U.; Bischof, J. Structure, regulation, and (patho-)physiological functions of the stress-induced protein kinase CK1 delta (CSNK1D). Gene 2019, 715, 144005. [Google Scholar] [CrossRef]
- Gallego, M.; Kang, H.; Virshup, D.M. Protein phosphatase 1 regulates the stability of the circadian protein PER2. Biochem. J. 2006, 399, 169–175. [Google Scholar] [CrossRef]
- Lee, H.; Chen, R.; Kim, H.; Etchegaray, J.P.; Weaver, D.R.; Lee, C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc. Natl. Acad. Sci. USA 2011, 108, 16451–16456. [Google Scholar] [CrossRef]
- Smadja Storz, S.; Tovin, A.; Mracek, P.; Alon, S.; Foulkes, N.S.; Gothilf, Y. Casein kinase 1δ activity: A key element in the zebrafish circadian timing system. PLoS ONE 2013, 8, e54189. [Google Scholar] [CrossRef]
- Song, H.; Pu, J.; Wang, L.; Wu, L.; Xiao, J.; Liu, Q.; Chen, J.; Zhang, M.; Liu, Y.; Ni, M.; et al. ATG16L1 phosphorylation is oppositely regulated by CSNK2/casein kinase 2 and PPP1/protein phosphatase 1 which determines the fate of cardiomyocytes during hypoxia/reoxygenation. Autophagy 2015, 11, 1308–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.J.; Logunova, L.; Maywood, E.S.; Gallego, M.; Lebiecki, J.; Brown, T.M.; Sládek, M.; Semikhodskii, A.S.; Glossop, N.R.J.; Piggins, H.D.; et al. Setting clock speed in mammals: The CK1ε tau mutation in mice accelerates the circadian pacemaker by selectively destabilizing PERIOD proteins. Neuron 2008, 58, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, K.; Vallone, D.; Gondi, S.B.; Santoriello, C.; Dickmeis, T.; Foulkes, N.S. Temperature regulates transcription in the zebrafish circadian clock. PLoS Biol. 2005, 3, e351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Boronat, M.; De Pedro, N.; Alonso-Gómez, Á.L.; Delgado, M.J.; Isorna, E. Nuclear receptors (PPARs, REV-ERBs, RORs) and clock gene rhythms in goldfish (Carassius auratus) are differently regulated in hypothalamus and liver. Front. Physiol. 2022, 13, 9037799. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xia, H.B. Progresses on nuclear receptor Rev-erbs. Life Sci. Res. 2013, 17, 548–553. [Google Scholar] [CrossRef]
- Bugge, A.; Feng, D.; Everett, L.J.; Briggs, E.R.; Mullican, S.E.; Wang, F.; Jager, J.; Lazar, M.A. Rev-Erbα and Rev-Erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012, 26, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Zhang, F.; Ye, Q.; Wang, H. The circadian clock regulates autophagy directly through the nuclear hormone receptor Nr1d1/Rev-Erbα and indirectly via Cebpb/(C/Ebpβ) in zebrafish. Autophagy 2016, 12, 1292–1309. [Google Scholar] [CrossRef] [Green Version]
- Lazado, C.C.; Kumaratunga, H.P.S.; Nagasawa, K.; Babiak, I.; Giannetto, A.; Fernandes, J.M.O. Daily rhythmicity of clock gene transcripts in atlantic cod fast skeletal muscle. PLoS ONE 2014, 9, e99172. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Li, Y.L.; Cheng, J.; Chen, L.; Zhu, X.; Feng, Z.G.; Zhang, J.S.; Chu, W.Y. Daily rhythmicity of clock gene transcript levels in fast and slow muscle fibers from Chinese perch (Siniperca chuatsi). BMC Genom. 2016, 17, 1008. [Google Scholar] [CrossRef] [Green Version]
- Figueredo, D.d.S.; Barbosa, M.R.; Gitaí, D.L.G.; de Andrade, T.G. Predicted microRNAs for mammalian circadian rhythms. J. Biol. Rhythms 2013, 28, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, K.; Chen, H.; Zhao, M.; Ji, G.; Zhang, Y.; Cao, H.; Kan, G.; Li, Y.; Qu, L. Functional annotation of extensively and divergently expressed miRNAs in suprachiasmatic nucleus of ClockΔ19 mutant mice. Biosci. Rep. 2018, 38, BSR20180233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, M.V.; Hall, C.; Jury, A.; Crosier, K.; Crosier, P. The zebrafish retinoid-related orphan receptor (Ror) gene family. Gene Expr. Patterns 2007, 7, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Masana, M.I.; Sumaya, I.C.; Becker-Andre, M.; Dubocovich, M.L. Behavioral characterization and modulation of circadian rhythms by light and melatonin in C3H/HeN mice homozygous for the RORβ knockout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2357–R2367. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, M.; Sharma, A.R.; Sharma, G.; Patra, B.C.; Nam, J.S.; Chakraborty, C.; Lee, S.S. The crucial role and regulations of miRNAs in zebrafish development. Protoplasma 2017, 254, 17–31. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; He, K.; Liu, Q.; Luo, J.; Zhang, D.M.; Liang, J.; Liao, L.; Yang, S. MiRNA-mRNA integration analysis reveals the regulatory roles of miRNAs in the metabolism of largemouth bass (Micropterus salmoides) livers during acute hypoxic stress. Aquaculture 2020, 526, 735362. [Google Scholar] [CrossRef]







| Genes/miRNAs | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| 18S rRNA | GACTCTGGCGTGCTAACTA | CAATCTCGTGTGGCTGAAC |
| period3 (per3) | AAGTGGGGCAGGAAGATGAA | TTCTTCATCTCAGCCACCGT |
| casein kinase 1, delta b (csnk1db) | ACCACGAGGAACCCAAGACG | CACGCTCCATTCCAGACACC |
| RAR-related orphan receptor b (rorb) | ACACACTCCTGGGACTTCTG | CGAACTAACCGTAACCGCTG |
| nuclear receptor subfamily 1, group d, member 1 (nr1d1) | AGTGCATGTGTGTCAGAGGT | CACATTCACCCGCTCATCAG |
| nuclear receptor subfamily 1, group d, member 2 (nr1d2) | AGTGCATGTGTGTCAGAGGT | CACATTCACCCGCTCATCAG |
| miR-361-5p | TTATCAGAATCTCCAGGGGTCC | |
| miR-210 | CTGTGCGTGTGACATCGGCT | |
| m0044-5p | GTGTTCAGTCTGTTGGTCCGTCT | |
| Universal primer R | GTGCAGGGTCCGAGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.-X.; Liang, Y.-S.; Niu, S.-F.; Zhang, J.; Tang, B.-G.; Liang, Z.-B. Transcriptomic Analysis Reveals Circadian Rhythm Homeostasis in Pearl Gentian Grouper under Acute Hypoxia. Fishes 2023, 8, 358. https://doi.org/10.3390/fishes8070358
Wu R-X, Liang Y-S, Niu S-F, Zhang J, Tang B-G, Liang Z-B. Transcriptomic Analysis Reveals Circadian Rhythm Homeostasis in Pearl Gentian Grouper under Acute Hypoxia. Fishes. 2023; 8(7):358. https://doi.org/10.3390/fishes8070358
Chicago/Turabian StyleWu, Ren-Xie, Yan-Shan Liang, Su-Fang Niu, Jing Zhang, Bao-Gui Tang, and Zhen-Bang Liang. 2023. "Transcriptomic Analysis Reveals Circadian Rhythm Homeostasis in Pearl Gentian Grouper under Acute Hypoxia" Fishes 8, no. 7: 358. https://doi.org/10.3390/fishes8070358
APA StyleWu, R. -X., Liang, Y. -S., Niu, S. -F., Zhang, J., Tang, B. -G., & Liang, Z. -B. (2023). Transcriptomic Analysis Reveals Circadian Rhythm Homeostasis in Pearl Gentian Grouper under Acute Hypoxia. Fishes, 8(7), 358. https://doi.org/10.3390/fishes8070358